
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For each pair, indicate which type of spectroscopy would most easily distinguish between the pair and give the single piece of info that
would be different that supports your choice. Choices are MS, IR, UV, H NMR, C NMR.
Please explain
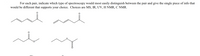
Transcribed Image Text:For each pair, indicate which type of spectroscopy would most easily distinguish between the pair and give the single piece of info that
would be different that supports your choice. Choices are MS, IR, UV, H NMR, C NMR.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- refer to image please please label graph and show all work. CALCULATE R VALUE AND EXPLAIN. will only rate if graph is labelled. label name of structure too.arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardWhich compound best fits this IR?arrow_forward
- Based on the spectra provided, draw the structure. Label each unique carbon and hydrogen with the letters A, B, C… for use in assigning NMR peaks. fill in the data table assigning peaks in each spectrum. You should assign: • All 1H NMR peaks • Significant IR peaks above 1600 cm Example of what the table should include (imagine the structure of ethanol is drawn with the CH3 hydrogens labeled as A, the CH2 hydrogens labeled as B, and the OH hydrogen labeled as C) : hydrogen proton chemical shift integration splitting pattern couples to.. A 1.2 3 triplet B B 3.7 2 quartet A C 2.6 1 singlet -arrow_forwardPlease determine the number of H-NMR spectrum signals. Thank youarrow_forwardhelp please answer in text form with proper working and explanation for each and every part and steps with concept and introduction no ai no copy paste remember answer must be in proper format with all workingarrow_forward
- For each pair, indicate which type of spectroscopy would most easily distinguish between the pair and give the single piece of info thatwould be different that supports your choice. Choices are MS, IR, UV, H NMR, C NMR. Please explainarrow_forwardPlease answer question 5,7 and 9 step-by-step thank you.arrow_forwardIdentify the following spectra. Full credit is only given if all pertinent peaks are assigned on the spectrum (~5-6 peaks). Label the peaks on the spectrum and place the structure of the compound in the box on the lower left-hand corner of the spectrum (from the table below, no numbering scheme). It is highly advisable to use the tables provided in the infrared handout and lecture to avoid confusion caused by tables from different sources. You do not have to indicate the exact wavenumber of the peak. please circle the peaks on the grapharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY