
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
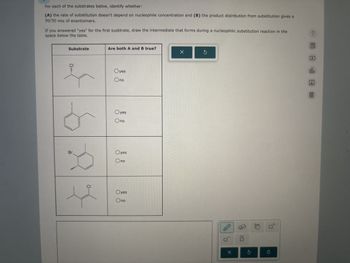
Transcribed Image Text:For each of the substrates below, identify whether:
(A) the rate of substitution doesn't depend on nucleophile concentration and (B) the product distribution from substitution gives a
50/50 mix of enantiomers.
If you answered "yes" for the first susbtrate, draw the intermediate that forms during a nucleophilic substitution reaction in the
space below the table.
Substrate
Are both A and B true?
Br.
P
Oyes
Ono
Oyes
Ono
Oyes
Ono
CI
Oyes
Ono
X
olo
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- For each of the substrates below, identify whether: (A) the rate of substitution doesn't depend on nucleophile concentration and (B) the product distribution from substitution gives a 50/50 mix of enantiomers. If you answered "yes" for the first susbtrate, draw the intermediate that forms during a nucleophilic substitution reaction in the space below the table. Substrate Are both A and B true? Oyes Ono Oyes Ono Br Oyes Ono Oyes Ono X G Click and drag to start drawing a structure. F 5 ס' P C B allaarrow_forwardFor each of the substrates below, identify whether: (A) the rate of substitution doesn't depend on nucleophile concentration and (B) the product distribution from substitution gives a 50/50 mix of enantiomers. If you answered "yes" for the first substrate, draw the intermediate that forms during a nucleophilic substitution reaction in the space below the tablearrow_forwardFor the following sets of substitution reactions that occur via an SN2 mechanism, draw the main organic substitution product (i.e., not the leaving group, for each reaction draw the product, though the products may be equivalent) and indicate which reaction occurs at the faster rate. (a) H3C CH₂O H3C E H3C Br (b) Br CH3O H3C + H3C-Br CH3O- H3C-Brarrow_forward
- Draw a structural formula for the major organic product of each reaction and specify the most likely mechanism by which each is formed. (g) CH3CH2ONa++CH2=CHCH2Clethanolarrow_forwardThe formation of Br2 from NBS first involves the reaction of NBS with HBr to form an iminol intermediate and molecular bromine. The intermediate then undergoes acid-catalyzed tautomerism to form succinimide, the byproduct of the reaction. Propose a curved-arrow mechanism for the conversion of NBS into succinimide that also accounts for the formation of Br2.arrow_forwardWhen performing a nucleophilic substitution reaction and not knowing the structure of the starting material or product, how can you form a method to determine whether the reaction proceeds via an SN1 or SN2 mechanism?arrow_forward
- Like acid chlorides, acid anhydrides react with alcohols in the presence of pyridine to form esters. For the following reaction : (a) Draw a circle around the nucleophile ; (b) Draw a square around the electrophile; (c) Put a star next to the base; (d) Draw the structure of the tetrahedral intermediate in the box below. Then draw a reasonable 3-step arrow pushing mechanism for the reaction.arrow_forwardUsing the pKa values of the conjugate acids of the leaving groups (the pKa of HBr is -9, and the pKa of H2O is 15.7), explain the difference in reactivity between CH3Br and CH3OH in a nucleophilic substitution reaction.arrow_forwardFor the reactions below predict the product that will form via the stated mechanism then explain why using the stated effect the reaction will proceed through the supplied mechanismarrow_forward
- The following substances can be prepared by a nucleophilic addition reaction between an aldehyde or ketoneand a nucleophile. Identify the reactants from which they were prepared. If the substance is an acetal, identifythe carbonyl compound and the alcohol; if it is an imine or enamine, identify the carbonyl compound and theamine. You do not have to consider stereochemistry. In cases where there is more than one answer, just giveone. Use Grignard reagents when an organometallic reagent is required. Draw the Grignard reagent as acovalent magnesium bromide.arrow_forwarda) Propose a stepwise synthesis for the compound below starting from benzene. List all reagents and intermediates. You can assume that ortho- and para- substituted benzenes can be separated efficiently. (3 reactions) Br O₂N b) Leveraging your knowledge of electrophilic aromatic substitution mechanisms, propose an arrow-pushing mechanism for the nitration step of the synthesis in a). Make sure to include all charges, intermediates, and the formation of the electrophile in this reaction. You can use benzene as a model reagent (see below). HNO3 H2SO4 NO2 c) Substituted benzyl halides can react with methanol via an SN1 mechanism to form benzyl ethers. List the benzyl halides below in order of increasing reactivity under these conditions (i.e., from the least reactive to the most reactive). Br II Br III Br IV Brarrow_forwardAn organic chemistry experiment involves a nucleophilic substitution (SN2) reaction using 1-iodobutane and 2-naphthoxide ion. The 2-naphthoxide ion is generated from 2-naphthol and sodium hydroxide in an ethanol solvent. The experiment includes steps like generating the nucleophile, conducting the SN2 reaction, and analyzing the product using 1H NMR spectroscopy. Question: Write the structure of the undesired side product if CH3CH2O‾ (ethoxide) reacts with 1-iodobutane.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning