
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
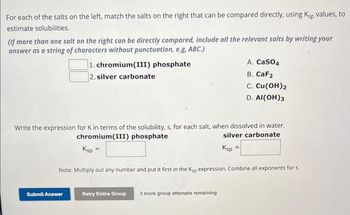
Transcribed Image Text:For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to
estimate solubilities.
(If more than one salt on the right can be directly compared, include all the relevant salts by writing your
answer as a string of characters without punctuation, e.g, ABC.)
1. chromium(III) phosphate
2. silver carbonate
Submit Answer
Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water.
chromium(III) phosphate
silver carbonate
Ksp =
Ksp =
Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s.
A. CaSO4
B. CaF2
C. Cu(OH)2
D. Al(OH)3
Retry Entire Group 3 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . Teeth and bones are composed, to a first approximation, of calcium phosphate. Ca3(PO4)2(s). The K for this salt is 1.31032at 25 °C. Calculate the concentration of calcium ion in a saturated solution of Ca3(PO4)2.arrow_forwardFor each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities. (If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) Ksp 1. barium phosphate 2. chromium(III) phosphate Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. barium phosphate chromium(III) phosphate Ksp= DENG A. ZnS B. Pb(OH)2 C. Zn3(PO4)2 D. PbCrO4arrow_forwardFor each of the salts on the left, match the salts on the right that can be compared directly, using Köp values, to estimate solubilities. (If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) manganese(II) hydroxide 2. iron(III) hydroxide Ksp 1. manganese(II) hydroxide = Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. A. Ag₂SO4 B. AgCl C. Cr(OH)3 D. Cas Ksp iron (III) hydroxide Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s.arrow_forward
- For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities. (If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) Ksp 1. calcium chromate 2. lead phosphate Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. calcium chromate lead phosphate = Ksp A. Zn3(PO4)2 B. PbI₂ C. NICO3 D. BaSO3 = Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s.arrow_forwardPlease don't provide handwritten solutionarrow_forwardWhich compound in each of the following pairs of compounds is the more soluble one? Solubility Product Constants, , at 25°C Substance Formula Ksp Strontium chromate 3.5x10^-5 Strontium carbonate 9.3x10^-10 Magnesium hydroxide 1.8x10^-11 Copper(II) hydroxide 2.6x10^-19 strontium chromate or strontium carbonate strontium carbonatestrontium chromate magnesium hydroxide or copper(II) hydroxide copper(II) hydroxidemagnesium hydroxidearrow_forward
- Is Ag2SO is added to water ,the salt will be?. Soulable or insoulable? Explain why?arrow_forward4.) The K,= 1.46×10-º for CaF,. Suppose you add 0.080M of NaF. Use an I.C.E. table to calculate the molar solubility_(S), in the presence, of a common ion (F=").arrow_forwardCadmium ion in solution is analyzed by precipitation as thesulfide, a yellow compound used as a pigment in everythingfrom artists’ oil paints to glass and rubber. Calculate the molarsolubility of cadmium sulfide at 25C.arrow_forward
- Why will silver hydroxide be the least soluble in a solution of higher ph?arrow_forwardThe molar solubility of lead carbonate in a 0.251 M ammonium carbonate solution is M.arrow_forwardHow many liters of pure water willbe required to dissolve the same amount of solid Lead Iodate (0.885g), if the water already intially contains .135M NaIO3?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co