
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For each of the following, identify the product(s) that would be formed through the indicated sequence of steps from the given starting material.
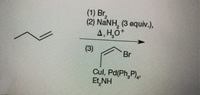
Transcribed Image Text:(1) Br,
(2) NANH, (3 equiv.),
4, H,O+
(3)
Br
Cul, Pd(Ph,P),
Et,NH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation neededarrow_forwardSuggest synthetic routes to each of the following form the indicated starting materials. You may use any other reagents. You can use other organic starting materials that is not explicitly excluded. However. all the carbons in the indicated starting materials have to end up in the final products.arrow_forwardBased on the following seven-step synthetic scheme, answer the questions below. Indicate, in the schematic, the reagents you would use in each are available in the following inventory reagents from an FDA lab. NaCl, CH3OH, terbutanol, K, Na, K2SO4, THF, TsCl, NaOCH3, B2H6, H2O, H3PO4 85%, H2O2, H2O nanopura, PCC, K2Cr2O7, H2SO4, Mg, CH3Br, Cl2, NaOH, HBr 5%, HBr conc., fenolftaleína, dextrina, NaF, KMnO4, HCl 1%, CaCO3, C2H2, C6H6, H2, Pt, CCl4arrow_forward
- What is the purpose of CH3OH being the solvent? Isn't CH3S- the only reagent needed in order to go through an SN2 reaction with the replacement of the bromine?arrow_forwardThe following alkene is treated with one equivalent of NBS in CH2Cl2 in the presence of light to give bromination products. Please draw the structural formula for each product formed. Please it’s my last chance so it has to be right. Thank you!arrow_forwardPropose a synthesis of Compound R from the starting materials provided. NOTE: Your only sources of carbon in the final product are the starting materials on the left. It is acceptable to have other carbon sources in reagents and protecting groups. You may assume that you have the starting materials in separate bottles and can react them separately. NOTE: Remember, you can define R groups. CEN OH Compound Rarrow_forward
- An example of the McFayden-Stevens reaction is shown below, in which an acyl chloride is converted to an aldehyde. First, benzoyl chloride is reacted with hydrazine, H2NNH2, the product of which is reacted with benzenesulfonyl chloride. The result is a 1-benzoyl-2-benzenesulfonylhydrazide, which, when heated under basic conditions, decomposes into the aldehyde. Provide the detailed mechanism showing the conversion of benzoyl chloride into 1-benzoyl-2-benzenesulfonylhydrazide. H 1. H,NNH2 Na,CO3 CI `SO,C;H5 2. CgHgSO2CI A Benzoyl chloride 1-Benzoyl-2-benzenesulfonylhydrazide Benzaldehydearrow_forwardSuggest synthetic routes to each of the following form the indicated starting materials. You may use any other reagents. You can use other organic starting materials that is not explicitly excluded. However. all the carbons in the indicated starting materials have to end up in the final products.arrow_forwardWhich statement best characterizes the following reaction of 2-phenylpropan-2-ol with HBr? OH CH3 CH3 + HBr Br CH3 CH3 + H₂O It is an SN1 reaction with bromide as the nucleophile and water as the leaving group. It is an SN1 reaction with HBr as the nucleophile and hydroxide ion (HO-) as the leaving group. It is a one step concerted reaction with bromide as the nucleophile and hydroxide ion (HO-) as the leaving group. It is an SN2 reaction with bromide as the nucleophile and water as the leaving group.arrow_forward
- 4. Give the mechanism for the following reaction of a Grignard reagent (RMgBr) with diethylcarbonate.arrow_forwardAnother mechanism for the formation of epoxides is through the formation of a chlorohydrin. Alkenes react with chlorine in the presence of H₂O to give a chlorohydrin via a cyclic chloronium ion intermediate. When the chlorohydrin is treated with strong base, HCI is eliminated and the epoxide is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions ~A 1. Cl₂, H₂O 2. NaOH Ci: XII OH O H₂O :CI: llaarrow_forwardDraw the product that is expected when each of the following compound undergoes a ring-closing metathesis (RCM). Include stereochemistry in your answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY