
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Thank you!

Transcribed Image Text:Tes
Identifying Bronsted-Lowry acids and bases
For each chemical reaction in the table below, decide whether the highlighted reactant is a Brønsted-Lowry acid, a Brønsted-Lowry base, or neither.
highlighted reactant
reaction
Bronsted-Lowry
acid
Bronsted-Lowry
base
neither
+
HBr(aq) + NH,(aq)
Br (aq) + NH, (aq)
HBr(aq) + NH,(aq)
Br (aq) + NH (aq)
Br (aq) + NH (aq) → HBr(aq) + NH,(aq)
4
Br (aq) + NH (aq)
HBr(aq) + NH3(aq)
4
||
Expert Solution
arrow_forward
Step 1
Bronsted-Lowry acid-base theory:
According to the Bronsted-Lowry acid-base theory, acid is a substance that gives H+ ions and base is a substance that accepts the H+ ions.
The species which are differing by the H+ called conjugate acid-base pairs.
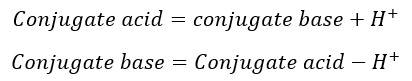
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why should you wear black in the winter and white in the summer? should be an original thought, not Google’s 3-4 sentences asap please!! will rate!! Thank you!!arrow_forwardWhich pack had the greatest change in enthalpy? How do you know?arrow_forwardAnswer provided: 2.5% Al2(SO4)3 Please show your complete solution and write your answer clearly and readable. Thank you.arrow_forward
- in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forwardWe want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forward00 %24 II Assessment Assignment Score: Resources Give Up? O Hint Check Answer 75% McQuarrie Rock Gallogly presented by Macmillan Learning Glucose, C,H,O6, is used as an energy source by the human body. The overall reaction in the body is described by the equation 12 (1DOʻH9 + (3) 0Ɔ9 – (8)09 + (be)'0H°Ɔ Calculate the number of grams of oxygen required to convert 23.0 g of glucose to CO, and H,O. mass of O2: Calculate the number of grams of CO, produced. mass of CO,: # 5 Marrow_forward
- Paraphrasing Tool | QuillBot AI X b Answered: Part A Classify each x Course Home Dashboard UC 6 Yoga Asanas To Help You Bur X openvellum.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=6acab9256cb89b6deOb7314be966bc5a#10001 Apps Yahoo Mail YouTube Maps Best Free PowerP... Google Drive on Academic Search Downloads € University Librarie... E UNIVERSITY POR... Student Detail Sc... > I Review I Constants I Periodic Table Scores Balance each of the following by determining coefficients, and identify the type of reaction. eТext Part A Document Sharing Identify the coefficients in the reaction: User Settings Course Tools > Enter your answers in order from left to right numerically separated by commas. Use the lowest possible coefficients. ? Submit Request Answer Part B Identify the type of reaction in Part A. combination double replacement combustion decomposition P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions |…arrow_forwardWe want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forward_engine.html?ClassID=D1746475745#3 Drie. 0.700 g of an impure FeCl3 sample are dissolved in water to create 50.0 mL of solution. It took 1.54 x 10-3 mol H2C204 to reach the equivalence point. 2FE3+ (aq) + H2C204(aq) CO2(g) + 2H*(aq) + 2FE2+(aq) -> How many moles of FeCl3 react? 1? [? ]x 10 mol FeCl3 Coefficient (green) Exponent (yellow) Enterarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY