
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
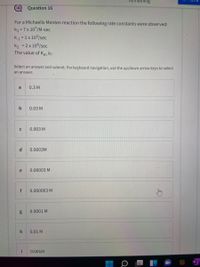
Transcribed Image Text:Question 16
For a Michaelis Menten reaction the following rate constants were observed:
k1 = 7x 107/M-sec
k1 =1x 103/sec
k2 = 2 x 104/sec
The value of Km is:
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select
an answer.
a
0.3 M
b
0.03 M
0.003 M
d.
0.0003M
e
0.00003 M
f
0.000003 M
0.0001 M
0.01 M
i
0.001M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Two solutions, 250.0 mL of 1.00 M CaCl2(aq) and 250.0 mL of 1.00 M K2SO4(aq), are combined, and the temperature decreased by 2.40 degrees C. Determine qrxn per mole of CaSO4(s) formed in the reaction. A) +12.0 kJ/mol B) -12.0 kJ/mol C) +6.00 kJ/mol D) -6.00 kJ/molarrow_forwardCalculate the average charge of H3A+ at pH 5.10 (pKa = 2.1, 3.9, 9.8). (Hint: remember that the answer must be ± 0.5 of the dominant charge.)arrow_forwardCalculate the Redlich-Kwong parameters of fluorine from the values of the critical constantsarrow_forward
- The reaction quotient is Q=1.6×10-26 Part B What pH is needed to produce this value of Q if the concentration and pressure values are [Br2]=2.50×10−4M , [Br−]=11.65M, [SO42−]=9.50M, and PSO2=3.50×10−5atm ? Express your answer numerically to two decimal places.arrow_forwardConsider the following reaction and its equilibrium constant: 12(g) 21(g) Kp = 0.209 atm A reaction mixture contains 0.89 atm 12 and 1.77 atm I. Which of the following statements is TRUE concerning this system?arrow_forwardGiven the following data, determine the rate law for the reaction below. 2H2 (g) + 2NO(g) → 2H2O(g) + N2(g) Experiment 1 23 [H2] (M) [NO] (M) Initial Rate (M/s) 0.0369 0.0262 0.0248 0.1107 0.0262 0.0774 0.0369 0.0524 0.0992 Rate = k[H2][NO] Rate = k[H2][NO] Rate = k[NO]2 Rate = k[H2][NO]² Rate = k[H2][NO]arrow_forward
- The units for the unimolecular reversible rate constant for the forward reaction are ○ M sec-1 sec M-1 sec-1 sec-1arrow_forwardWhen the following equation of a redox reaction in acidic solution is properly balanced, what are the coefficients for Cr2O72–, Fe2+ H+, Cr3+, Fe3+, and H2O, respectively? __Cr2O72– + __Fe2+ + __H+ --> __Cr3+ + __Fe3+ + __H2O (A) 1, 3, 14, 2, 3, 7; (B) 1, 6, 14, 2, 6, 7; (C) 2, 10, 14, 2, 10, 7; (D) 2, 12, 28, 4, 12, 14arrow_forwardUse the References to access important values if needed for this question. For the following reaction, 6.58 grams of water are mixed with excess diphosphorus pentoxide. The reaction yields 21.0 grams of phosphoric acid. diphosphorus pentoxide (s) + water (1) phosphoric acid (aq) What is the theoretical yield of phosphoric acid? grams What is the percent yield for this reaction? % Submit Answer Retry Entire Group more group attempts remaining 91arrow_forward
- AG°xn=-28.6 kJ Given the following equation, H2O(g) + CO(g) → H2(g) + CO2(g) Calculate AG°n for the following reaction. -> 8 H2O(g) + 8 CO(g) 8H2(g) + 8 CO2(g) a -71.5 kJ b -3.57 kJ C +3.57 kJ d +228.8 kJ e -228.8 kJ O O O O Oarrow_forwardCalculate AG for the below balanced redox reaction as written. You may find a list of standard reduction potentials useful. 3 12 (s) + 2 Al (s) → 61 (aq) + 2 Al3+ (aq) O-4.24 x 102 kJ/mol O-6.48 x 102 kJ/mol O -2.12 x 102 kJ/mol O-1.27 x 103 kJ/mol O-6.37 x 102 kJ/molarrow_forwardCalculate the value of K for a reaction that has ΔG° = 37.6 kJ mol-1 at 37.0 °C. (R = 8.3145 J mol-1 K-1) in kj/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON