
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
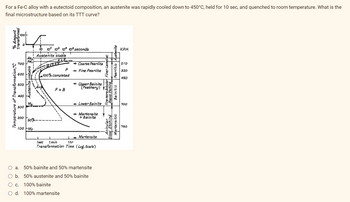
Transcribed Image Text:For a Fe-C alloy with a eutectoid composition, an austenite was rapidly cooled down to 450°C, held for 10 sec, and quenched to room temperature. What is the
final microstructure based on its TTT curve?
Amount
Temperature of Transformation, C
700
600
500
400
300
200
Ae₂!
Ae₁
Austenite unstable
Ms.
10 10² 103 104 105 seconds
Austenite stable
50%-
100 MF
TELEP
P
100% completed
F+B
Coarse Pearlite
- Fine Pearlite
-Upper Bainite
(Feathery)
Lower Bainite
→ Martensite
+ Bainite
Martensite
1
1sec 1 min
1hr
Transformation Time (Log. Scale)
O a. 50% bainite and 50% martensite
O b.
50% austenite and 50% bainite
O c.
100% bainite
O d. 100% martensite
Finer Lamellac
Bainitic Pearlitic Austenite
Fine more Acicular
Rapid Etching
Martensitic
cicular,
Slow Etching
V.P.H.
210
320
450
700
750
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Calculate the amounts of solid phases of ferrite and cementite present in pearlite.arrow_forwardQI/ Draw a thermal equilibrium diagram for the binary alloy system (Si –Au), from the following data:- a- Si melts at 1414 °C, and Au melts at 1064 °C , and identify all phases are present in the diagram. b- Eutectic is formed at 360°C containing 20 Wt% Si -80 Wt% Au . c- Determine the amount of each phase for the alloy which consist 60 Wt% Si- 40Wt % Au at 1200 °C and 800 °C ,then determine the amount of eutectic at 200 °C?arrow_forwardQuestion 4 A segment of the Fe/C alloy phase diagram is shown below: Composition (at% C) 15 Temperature (°C) 0 1600 1400 1200 1000 800 600 400 1538°C 0 (Fe) 8 Y 912°C -1493 C 1394-C Y. Austenite 0.76 0.022 a, Ferrite 1 y+L 2 2.14 10 1147 C at Fe₂C L 4.30 Y+Fe3C 4 3 Composition (wt% C) 727°C 20 Cementite (Fe-C) 5 6 25 2500 2000 1500 1000 6.70 Temperature (°F) a) What are the equilibrium phases, compositions and relative fractions of the Fe-C alloy at an average composition Ccarbon of 2% and a temperature of 730 °C? b) What are the equilibrium phases, compositions and relative fractions of the Fe-C alloy at an average composition Ccarbon of 2% and a temperature of 725 °C? 7 c) Sketch the microstructure of the alloy obtained by quick cooling of the alloy from the conditions in part a) to the conditions in part b). Be sure to identify the phases in your sketch.arrow_forward
- Is it possible to have a copper-nickel alloy that, at equilibrium, consists of a liquid phase of composition 20 wt% Ni-80 wt% Cu and also an a phase of composition 37 wt% Ni-63 wt% Cu? If so, what will be the approximate temperature of the alloy? If this is not possible, explain why. Temperature (°C) 1600 1500 1400 1300 1200 1100 1000 0 1085°C (Cu) 20 Liquid Liquidus line L B 40 a+L a A 60 Composition (wt% Ni) (a) 1455°C Solidus line 80 2800 2600 2400 2200 2000 100 (Ni) Temperature (°F) Temperature (°C) 1300 1200 20 Liquid Tie line. a + Liquid a 1 KR I 1 1 B ↑ Co a + Liquid -S 30 CL Composition (wt% Ni) (b) 40 1 Ca a 50arrow_forwardthis is material science questionarrow_forwardMaterial science Assuming this system forms a laminar type eutectic, determine the volume proportion of phases for an equilibrium solidified 50% Pb alloy. Sketch the expected microstructure.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY