Question
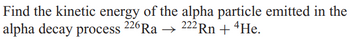
Transcribed Image Text:Find the kinetic energy of the alpha particle emitted in the
222 Rn + 4He.
alpha decay process 226 Ra
Expert Solution
arrow_forward
Concept and Principle:
- Alpha decay is one of the processes in which an unstable nucleus decays into a stable one. In alpha decay, the unstable nucleus disintegrates into a lighter nucleus and an alpha particle (nucleus of helium) is released.
- The following reaction takes place in alpha decay.
Here X and X' represent different nuclei.
- The kinetic energy of the alpha particle released is given by,
Here A is the atomic number of the parent nucleus and Q is the energy released in the reaction.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- Find the binding energy and binding energy per nucleon of the 27/13 Al nucleus.arrow_forwardComplete the following radioactive decay formulas: (a) B → ? + e¯ + D (b) 30 Th → Ra + ? (c) ? → 4N + e 88arrow_forwardFind the binding energy and the binding energy per nucleon of the 238-92 U nucleus. binding energy binding energy per nucleonarrow_forward
- If 88 (atomic number) 228 (atomic mass) ?? is produced, determine the parent isotope for each of the following types of decay and justify your answer. a) alpha decay b) beta negative decay c) beta positive decayarrow_forwardCobalt-60 undergoes beta minus decay. Determine the daughter nucleus and compare the calculated binding energy per nucleon of the parent and daughter nuclei. mass (Co-60) = 59.933817 u mass (daughter) = 59.930786 uarrow_forwardb) What type of decay is happening in the equation below? State two of the conservation laws that apply and explain how they are satisfied 238 92 U 234 Th + 4 a 90 2 c) Use the graph below to calculate the half-life of Protactinium 234, explain how you got your answer. Half Life of Protactinium 234 50 40 30 20 10 222222° 80 70 60 Activity (Bq) 0 20 20 40 40 60 80 100 120 140 160 Time (seconds)arrow_forward
arrow_back_ios
arrow_forward_ios