Question
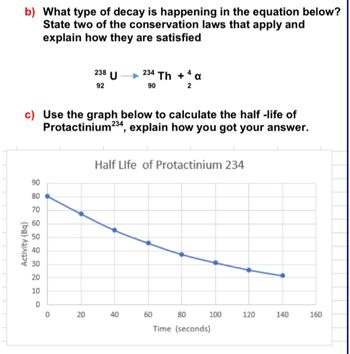
Transcribed Image Text:b) What type of decay is happening in the equation below?
State two of the conservation laws that apply and
explain how they are satisfied
238
92
U
234 Th + 4 a
90
2
c) Use the graph below to calculate the half-life of
Protactinium 234, explain how you got your answer.
Half Life of Protactinium 234
50
40
30
20
10
222222°
80
70
60
Activity (Bq)
0
20
20
40
40
60
80
100
120
140
160
Time (seconds)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- [Review Topics] [References] Radioactive vanadium-48 decays with a half-life of 16.0 days. a. What is the value of k in s12 k = b. A sample contains 58.0 mg 48 t° V. How many decay events will be produced in the first second? Assume the atomic mass of 48 V is 48.0 u. decays/s c. A chemist obtains a fresh sample of 8V and measures its radioactivity. She then determines that to do an experiment, the radioactivity cannot fall below 25% of the initial measured value. How long does she have to do the experiment? days Submit Answer Retry Entire Group 4 more group attempts remainingarrow_forwardRadon gas has a half-life of 3.83 days. If 2.92 g of radon gas is present at time t = 0, what mass of radon will remain after 2.20 days have passed? garrow_forwardc) Complete the following decay reaction to show sodium-24 undergoing ߯ decay. First identify the unknown daughter nucleus (?).. and then name the subatomic particle that completes the reaction. 24Na → (?) + v + (subatomic particle) Enter the mass number A = Enter the atomic number Z = Enter the chemical symbol: Name the subatomic particle: A/ Narrow_forward
- An unknown isotope has an atomic number, Z 15 and atomic mass number, A = 32. It decays via beta decay and emits an electron and antineutrino. %3D How many protons and neutrons are in the resulting daughter nuclide? o 16 protons 16 neutrons o 15 protons 17 neutrons o 13 protons 15 neutrons o 14 protons 17 neutrons o 14 protons 18 neutronsarrow_forwardPart A A sample of U (T1= 1.59 × 10° yr) contains 2 233 6.10 x 1018 nuclei. What is the decay constant? Express your answer using three significant figures. ? = s-1 IIarrow_forwardThe items on the right show an incomplete radioactive decay equation. Match the items at the left to correctly complete these equations. 266 1. 106Sg -> 2. 24°Cm 238U 23 Th + 90 96 3. He 23Pu + He → n + ón + ộn + 94 4. -je 266 106 → öY +arrow_forward
- Determine which decays can occur spontaneously. (a) Ca → e* + K (b) Ru → He + Mo 19 44 42* (c) 144Nd → He + 140 Ce 60 58arrow_forwardWhat type of decay is happening in the equation below? State two of the conservation laws that apply and explain how they are satisfied. 238 234 4 U ---> Th + (alpha) 92 90 2arrow_forwardAn unlabeled container of radioactive material has an activity of 78 decays/min. Four days later the activity is 63 decays/min. A. Determine the half-life of the material. T = ? days B. How many days after the activity is 78 decays/min will it reach 10 decays/min? t = ? daysarrow_forward
- a) What is the decay constant of fluorine-17 if its half-life is known to be 66.0 s? λ = b) How long will it take for the activity of a sample of 17F to decrease to 80% of its initial value? t80% =arrow_forwardWhat is the activity of a sample of C that contains 5.3x1020 nuclei? The half-life of C is 5700 years. Express your answer using two significant figures. ? ΔΝ decays/s Atarrow_forward238U is long-lived but ultimately unstable; it will eventually spontaneously break into two fragments, a 4He nucleus and a 234Th nucleus, in a process called alpha decay, which we’ll learn about in the next section. A great dealof energy is released in the process. What must be true about the masses of the nuclei involved?A. mU > mTh + mHeB. mU = mTh + mHeC. mU < mTh + mHearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios