
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
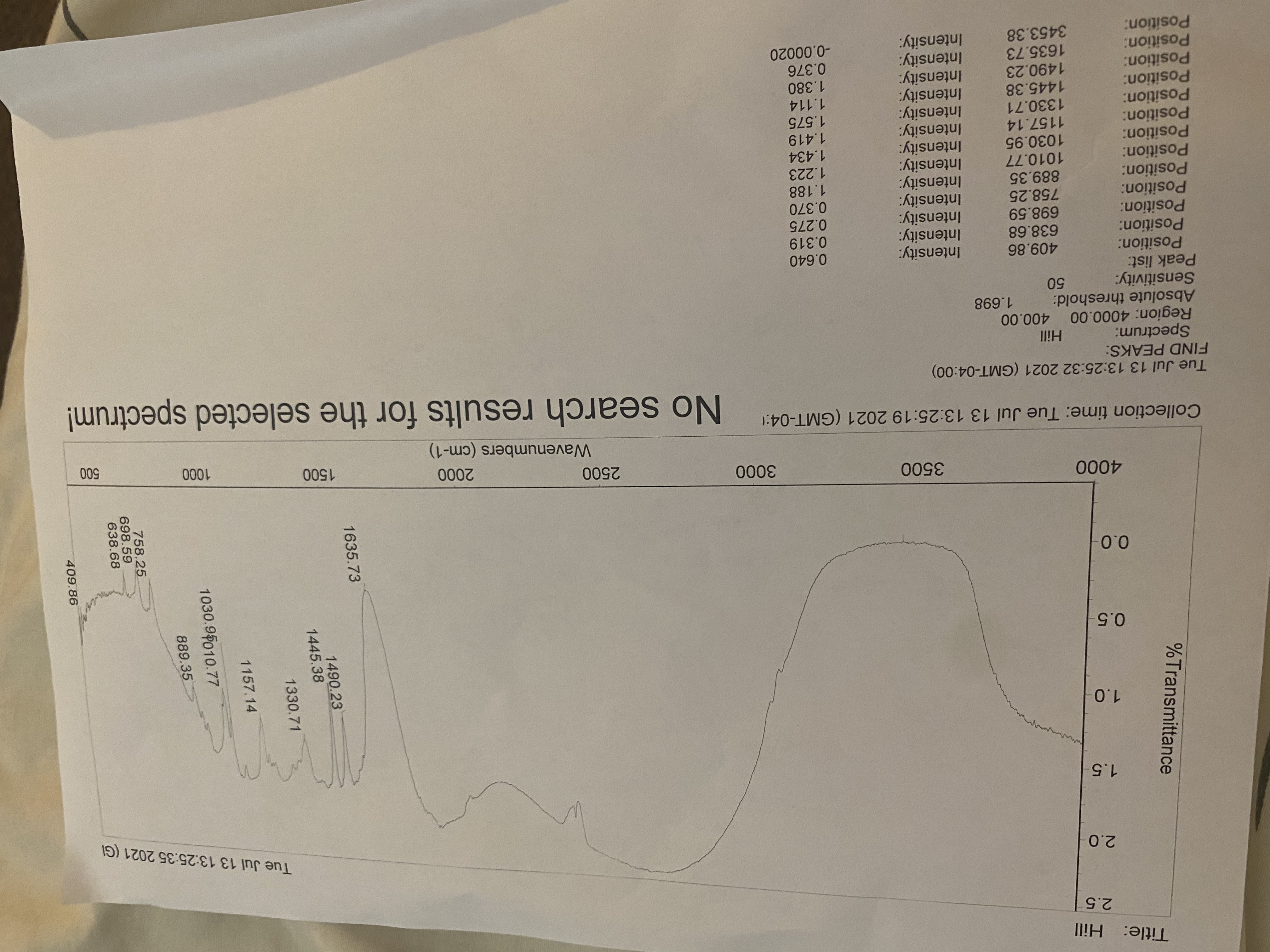
Fill the table base on data:
Lit.Methyl Banzoate.
Lit. Product
Experimental product

Transcribed Image Text:MgBr
THE
ОН
H,0
HCI
2 equiv
Phenylmagnesium Bromide
Methyl Benzoate
Triphenylmethanol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following: A. n-heptane B. 2-methyl-2-ethylbutane C. 3,3-dimethylpentane D. 2,2-diethylpropane E. none of thesearrow_forward1. The following molecule contains the functional groups A. secondary amine, secondary alcohol, ketone, carboxylic ester B. amide, primary alcohol, aldehyde, carboxylic acid C. secondary amine, two secondary alcohols, and two ketones D. primary amine, secondary alcohol, ketone, and carboxylic acid The following molecule contains the functional groups 2. A. primary alcohol and primary amine C. secondary alcohol and primary amine 3. A. alkene and secondary alcohol C. ketone and seconary alcohol The following molecule contains the functional groups B. ether and ketone D. ether and alkene 4. The following molecule A. primary amine and ketone C. secondary amine and alkyne NH₂ contains the functional groups B. secondary amine and alkene D. secondary amine and ketone ОН OH B. secondary alcohol and secondary amine D. primary alcohol and secondary amine NH₂ OHarrow_forwardWhat functional groups are contained in the molecule below? methyl amino phosphate carbonyl carboxyl hydroxylarrow_forward
- draw only using full structural formula, draw only using full structural formula, draw only using full structural formula pleasearrow_forwardIdentify the functional groups in the reactants. Choose all that apply. a. primary alcohol b. aromatic c. tertiary alcohol d. phenol e. ester f. carboxylic acid g. aldehyde h. secondary alcoholarrow_forwardUser Guard™ What kind of functional group is hydrolyzed to form soap? A. Aldehyde B. Amine C. Acid chloride D. Ester Q Search ****** ******* ********* *** app.honorlock.com 99+arrow_forward
- a. CI NaOH. !!!OH EZONLY 애arrow_forwardselect all of the following words that describe the features of the following. a. 1 alcohol b. amine c.alkene d.halide e.2 alcohol f.ketone g. ester h.ether i.3 alcohol j.aldehyde k.epoxidearrow_forwarddraw the structural formula... ethoxycyclobutanearrow_forward
- Determine the functional group(s) for the following molecule (choose all that apply).CHOOSE ALL THAT APPLY a. aldehyde b. alcohol c. aromatic d. ketone e. carboxylic acid f. hemiacetal g. acetalarrow_forwardHome the Structure Question 46 Part A Draw the structure of the organic product of the reaction between benzoic acid and ethanol. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atc EXP. CONTI C CI Marvin JS Br [1] by ChemAxon SI P. F.arrow_forwardname the following: A. n-heptane B. 2-methyl-2-ethylbutane C. 3,3-dimethylpentane D. 2,2-diethylpropane E. none of thesearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY