
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Complete rest of table
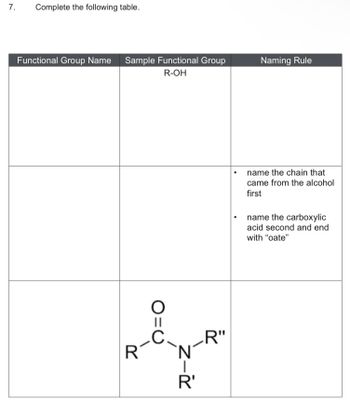
Transcribed Image Text:7. Complete the following table.
Functional Group Name Sample Functional Group
R-OH
R
||
I
R'
R"
Naming Rule
name the chain that
came from the alcohol
first
name the carboxylic
acid second and end
with "oate"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- be Maps Netflix in other Co... Nhttps://lms.naz.ed... Rochester to Seat... rus What is the mass of one formula unit of calcium cyanide, Ca(CN)2? amu Question #6 t How would you calculate (see below) the number of formula units of Ca(CN)2 of in 7 moles of Ca(CN)2? C V port 7 x formula mass (A) 7 x molar mass (B) 7 x Avogardro's Number (C) 7 7 formula mass (D) molar mass (E) Submit Answers 7 Avogardro's Number (F) McKee Next Previous 60arrow_forward(4) question is in the photo belowarrow_forwardHelp pleasearrow_forward
- M Apple Google Disney ESPN Yahoo! Biomedical Careers Program ☆ For the reaction Fe(s) +2HCl(aq)→→→→→FeCl₂(s) + H₂(g) AH° = -7.4 kJ and AS° = 107.9 J/K B Submit Answer Apple ☆ prod03-cnow-owl.cengagenow.com iCloud Yahoo Images Bing Google Wikipedia Facebook Twitter LinkedIn G b COWLv2... b D2L D2L D2L [Review Topics] [References] Use the References to access important values if needed for this question. D2L The maximum amount of work that could be done when 2.08 moles of Fe(s) react at 286 K, 1 atm is Assume that AH° and AS° are independent of temperature. Retry Entire Group 4 more group attempts remaining The Weather Channel C D2L Yelp TripAdvisor M kJ. +arrow_forwardQUESTION 14 LİCH,CH, HO i) ii) iii) iv) HO D Pr D. D. Pr НO Pr HO Pr O ivarrow_forwardCase Study - Bottling a Carbonated Drink The process of dissolving carbon dioxide (CO,) in water in known as carbonation. The presence of this gas creates bubbles and fizzing in the water. This process can occur naturally in some instances (e.g, beer) or artificially by injecting the C0, gas into the water under pressure. The process for artificially dissolving CO, was discovered in the 1776 but wasn't commercialized until 1807. Since then, the market for carbonated drink has consistently grown. Currently, carbonated drink can be found all over the world and are typically packaged using metals (aluminum), ceramics (soda-lime glass) and polymers (plastic #1). Perform an analysis of each of these materials. List the pros and cons of each and ultimately determine which of these materials is best considering the requirements listed below. 1) Keep the CO, - who wants to purchase a flat soda 2) Be non-toxic and chemically unreactive- this is meant for human consumption 3) Be inexpensive -…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY