
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
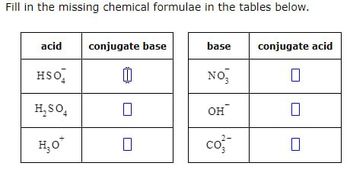
Transcribed Image Text:Fill in the missing chemical formulae in the tables below.
acid
conjugate base
base
conjugate acid
HSO
NO₁₂
☐
H₂SO
☐
он
☐
H₂O
co
☐
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- C+½ 020l co CO (s) 2 (g) CO+ 2 02(a) (g) AH = - 111 kJ %3D CO, 2 (g) Is this reaction endothermic or exothermic? (g) AH = - 394 kJ %3D -11 K3+(-394 C6st O2 (9)-yCO26) AH=-505 KJ Exothermic 2. a) Explain how AT would be affected if a greater amount of surrounding solvent (water) is used, assuming the mass of salt remains constant? b) Explain how qien would be affected if a greater amount of surrounding solvent (water) is used? Explain. If the following enthalpies are known: AH = - 95KJ 2 C+D A+2B- C) AH = + 50kJ B+Xarrow_forwardkJ H2(g) + 0.502(g) → H₂O(g) AH-241.8- AS-44.5 J-mol¯-K-¹ mol So, mol¹KI החומר ??? H₂O(®) 130.7 H2(g) 205.2 02(g)arrow_forwardGiven the following data C,H,(9) + 30,(9) → 2 CO,(g) + 2H,0(1) 2C,H6 (g) + 702(g) → 4 CO2(g) + 6H2O(1) AH=-3119.8 kJ 2H,(g) + O2(9) → 2H,O(1) AH= -1411.0 kJ AH=-571.7 kJ calculate A H for the reaction C2H4 (9) + H2 (g) → C,H6 (g) AH = kJarrow_forward
- Did I do this correctly?arrow_forwardwhat are the coefficients missing on the following equation?_KN03+_H2CO3--->_K2CO3+_HNO3 O (2,1,1,2) O (0,0,0,2) (2,2,0,0) O (0,0,0,0) O (2,2,2,0)arrow_forwardName these organic compounds: Η H H H | | | Η· C C C C C=H | | | | | H H H H H — H Η structure — — H H - C C 1 1 Η - Η - - Η - H | H — C - Η | H namearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY