
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Answer choices:
Blank 1-
a.
b. proton
Blank 2-
a. alkene
b. proton
Blank 3-
a. the carbocation
b. water
Blank 4-
a. the carbocation
b. water
Blank 5-
a. water
b.proton
Blank 6-
a. water
b.proton
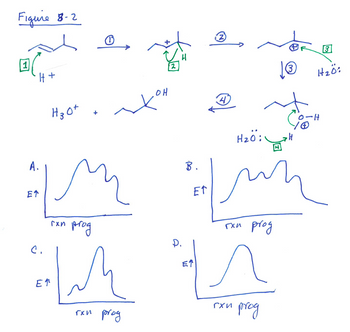
Transcribed Image Text:Figure 8-2
mee
1
B3 (4+
A.
ET
C.
ET
H30+
+
rxn
prog
Ich
rxn prog
OH
P.
B.
Et
ET
2
H₂O:
ГХИ
13
inn
m
гхи prog
prog
H₂ő:
![Figure 8-2 shows a mechanistic scheme for an organic reaction.
In Step 1, the nucleophile is [Select ]
[Select]
In Step 3, the nucleophile is [Select ]
[Select]
In Step 4, the nucleophile is [Select]
[Select]
and the electrophile is
and the electrophile is
and the electrophile is](https://content.bartleby.com/qna-images/question/03317af0-4144-4b0a-a13c-c65183a22c1d/615c375b-e1c8-44b2-9266-32437d01f1f4/8ip4u5g_thumbnail.png)
Transcribed Image Text:Figure 8-2 shows a mechanistic scheme for an organic reaction.
In Step 1, the nucleophile is [Select ]
[Select]
In Step 3, the nucleophile is [Select ]
[Select]
In Step 4, the nucleophile is [Select]
[Select]
and the electrophile is
and the electrophile is
and the electrophile is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Organic chem 2 structuresarrow_forwardWhich reaction is correctly balanced? Group of answer choices H2O2 --> H2O + 2 O2 2 H2O --> 2 H2 + 2 O2 2 Na2O2 + 2 H2O --> 4 NaOH + 3 O2 2 KNO3 --> 2 KNO2 + O2arrow_forwardWhat is the correct Lewis structure for acetaldehyde, an aldehyde which has the molecular formula CH,O? H :0 :0 H- H :0: H :0: H -č: FC-H H- -H H- -C-H H- C -ċ-H H- H H H a b d e a. Ob. Oc. Od. Oe.arrow_forward
- R/S Isomers HN 6. COOH H3COC COC. 7. SH CONH₂ HN HO 8. HO 9. N CHO HO HS 10. COOHarrow_forwardWhat is the missing reactant? I OCH₂-OC₂H5 + II. Select one: O A. I B. IV O C. III D. II OH i III. NaOC₂Hs H*(aq) HOC₂H5 IV. -OC₂Hs Moim -NH₂ CH-C-OC₂Hsarrow_forwardWhat is the product of this reaction? NH2 2) H,0* OH B. A. OH CHO. D. O A OB MacBook Pro G Search or type URL $ & %23 4 5 6 7 8 R T Y Uarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY