
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please give naser and explain process
thank you
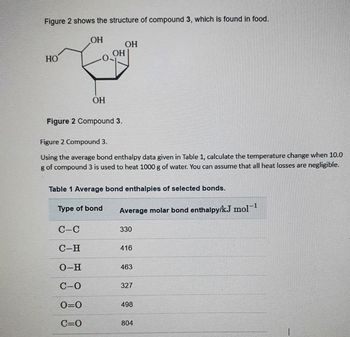
Transcribed Image Text:Figure 2 shows the structure of compound 3, which is found in food.
HO
OH
ОН
Figure 2 Compound 3.
C-C
C-H
O-H
C-0
0=0
C=O
OH
OH
Figure 2 Compound 3.
Using the average bond enthalpy data given in Table 1, calculate the temperature change when 10.0
g of compound 3 is used to heat 1000 g of water. You can assume that all heat losses are negligible.
Type of bond
Table 1 Average bond enthalpies of selected bonds.
Average molar bond enthalpy/kJ mol-¹
330
416
463
327
498
804
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4 ☆ www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIngUncfv-OChvG0b8him-_Mk4pOrDhiRiLH.... Reading Schedule 19.6 Reduction Po.... a SOLUTION: The le... Math 115 W-S Fall... h. Get O lubility and 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... OKINETICS AND EQUILIBRIUM Using first- and second-order integrated rate laws Consider this reaction: Nancy ! 1. CICH₂CH₂Cl (g) → CH₂CHC1 (g) + HCl (g) At a certain temperature it obeys this rate law. rate - (2.00 s¹) [CICH₂CH₂CI] Suppose a vessel contains CICH₂CH₂Cl at a concentration of 1.22M. Calculate how long it takes for the concentration of CICH₂CH₂Cl to decrease to 21.0% of its initial value. You may assume no other reaction is important. Round your answer to 2 significant digits. s A Explanation all N 2 Check W S X 3 H option command # E D 0.9 13 $ 4 C X > R F 5 % 5 V I T G tv N ^ 6 MacBook Pro B Y We 4 & 7 H U N Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy…arrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help A 84% Wed 8:14 T WPAL 101_ 233 _Spring 2022 O NEW BEST Builder Hall 5 Base x ALEKS - David Teague - Learn x X Grades for David Teague: CHM A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZI6tTytly4Fcfu6zOtOf80MM9sfGh3NIP72XeoPmXkrk4zqM9ylm-ZZhvViL. O ☆ D Paused O CHEMICAL REACTIONS David Writing a chemical equation from a description of the reaction 2/5 Aqueous potassium nitrate (KNO3) and solid silver bromide are produced by the reaction of aqueous potassium bromide and aqueous silver nitrate (AgNO,). Write a balanced chemical equation for this reaction.arrow_forward- Discord | #-courtyar → C Smail Official Miami Dade College x www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-UibwdleAUz2IYCcR4NM7yr_IpMriNwxObE8BNBktcRGBtTHK1XmgWvpNI8wuDkZtcqnDqEtC1XWgHvKTc... ☆ YouTube Translate O KINETICS AND EQUILIBRIUM Calculating the change in concentration after a whole number o... y! mdc.edu - Yahoo Search Resu X Hg mL Explanation 83°F Mostly sunny The rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half life of 28 minutes. Suppose in a particular patient the concentration of this drug in the bloodstream immediately after injection is 0.47 ug/mL. What will the concentration be 140 minutes later? Round your answer to 2 significant digits. Check x10 X H 5 MHCampus/Connect(ALEKS) X A ALEKS- Mia Reboredo - Lear X Q Search LO 4/5 + A k © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acc % 040 coarrow_forward
- ALEKS M Gmail T YouTube www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusKT1HM1Oky9642X811N-_5hQe-X8gueXU86ylJbno0NB3CBMB13myl-0dBa6q?10Bw7QYjl... ☆ Maps Translate time (minutes) KINETICS AND EQUILIBRIUM Deducing a rate law from the change in concentration over time A chemistry graduate student is studying the rate of this reaction: NH₂OH(aq) → NH3(aq) + H₂O (aq) He fills a reaction vessel with NH₂OH and measures its concentration as the reaction proceeds: 0 ALEKS - Rafia Riaz - Learn 10. Explanation 20. Type here to search 30. 40. News [NH₂OH] 0.800M 0.481 M 0.289 M 0.174M 0.104 M Write the rate law for this reaction. Use this data to answer the following questions. X b Answered: = O KINETICS AND EC X + Calculate the value of the rate constant k. College information Check 밥 rate = k x10 ロ・ロ * 4 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use (32) 1/3 Privacy Center 26°F Cloudy Rafia 唱 olo Ar Accessibility x Other bookmarks 9:24 AM…arrow_forwardHello! I am super confused on how to solve this problem. Any help is greatly appreciated! Thanks!arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
- + + HN in ethyl acetate (organic) treat with aqueous HCI Aqueous layer Organic layer treat with treat with aqueous NaOH; aqueous NaOH collect precipitate by filtration aqueous organic Final product #1 treat with "dry" with MgSO4, filter, evaporate ethyl acetate aqueous HCl; Be sure to label all glass containers of colorless solutions... collect precipitate by filtration It's easy to lose track! Final product #2 Final product #3arrow_forwardThe The O Cont Full Enha Dash MISS C Assu B The C This O Effec 5 Mc G syml college.com/course.html?courseld316985674&OpenVellumHMAC=Dad585d15954695d5b7068f8de43c2850#10001 View Available Hint(s) V2 = L Submit < Return to Assignment Provide Feedback 7:03 PM 76°F Partly cloudy 12/12/2021 19 rch 56 BHJC04Z022B01 E7arrow_forwardView Help ✓ FALT Paragraph Search Heading 1 Thermometer Fractionating column Water Normal 1. Show derivation for the Clausius-Clapeyron equation. 2. Draw a phase diagram for molecular Iodine I2. 3. What is the name and (technique) shown below? Describe how this piece of equipment separates substances. Heater Condenser Distilling flask 1 Water Receiving flask No Spacing Adapter 29 I Heading 2 Styles Titlearrow_forward
- ww-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQs_dp5pR4ENzvdYC-70kXyMz36BqJhw3sVP4xjb1Ky5m48FPmOU7SRya7UtV_M_GkvzGb Assignme... Connect P Course Home E Login | Student Veri... Bb Logout MyProgrammingLab Imported From IE O ITEC2110:Summ O KINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes In Initial rate Cia A certain reaction is first order in H, and first order in 1,. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [#,] [1-] initial rate of reaction x10 1.73 M 0.614 M 6.00 Xx 10 M/s | M/s 0.614 M 0.538 M M/s 2.10 M 0.505 M Privacy Terms of Use O2020 McGraw-Hill Education. All Rights Reserved. Check Explanationarrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help → C My Shelf | RedShelf Chapter 9 ppt - af X Part A >> rian's na's webp lease dfarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY