
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
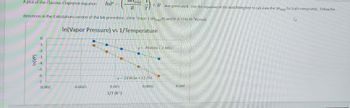
Transcribed Image Text:Avap
R
directions in the Calculations section of the lab procedure. (Hint: Slope-(-AHvap/R) and R-8.314x10 KJ/mol)
In(Vapor Pressure) vs 1/Temperature
A plot of the Clausius-Clapeyron equation
In(VP)
0
-1
-2
-3
-4
-5
ܗ ܂
0.002
0.0025
●
InP
de
-0.
0.003
1/T (K-¹)
T
+B was generated. Use the equation of the best fitting line to calculate the AHvap for both compounds. Follow the
4
y=-2930.6x + 7.4817
M
y=-5158.6x + 12.756
0.0035
10
0.004
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the appropriate values of Ksp and Kf from the table below to find the equilibrium constant for the reaction Express your answer to two significant figures. Keq= = VG ΑΣΦ 1.47.10⁹ CuS(s) + 4CN- (aq) = Cu(CN)4²¯ (aq) + S²- (aq) Ka (HS) 1 x 10-19 K₂ (HCN) 4.9 × 10-10 Ksp (Cus) 1.27 × 10-36 Kt([Cu(CN)6]4) 1 x 1025 Kf ([Cu(CN)4]²) 3 x 1018 Submit Previous Answers Request Answer X Incorrect; Try Again; 11 attempts remaining ovide Feedback 2- ?arrow_forwardtech Please don't provide handwritten solutionarrow_forwardshow-all-working-explaining-detailly-each-step Answer should be typewritten using a computer keyboard.arrow_forward
- 100ml of a saturated aqueous SrCrO4 solution contains 0.12 g of strontium chromate at 25°C. Calculate Ksp and deltaG° for the dissolving of strontium chromate in water.arrow_forwardCalculate the mean square displacement, (x²), of the H-Cl bond distance from its equilibrium positionarrow_forwardNonearrow_forward
- Ksp for BaCrO4 is 1.17x10^-10arrow_forwardphysical chemistry Roughly, what is the error introduced by the solution of some of the CO2 in the water in the bomb? The solubility of CO2 is about 0.0015 g/mL at 25°C and 1 atm CO2 pressure. The heat of solution of CO2 in a dilute aqueous solution is roughly -19.4 kJ/mol. Assume that the final CO2 pressure is 2 atm and that Henry's Law applies.arrow_forwardCalculate the theoretic delta H_sol (m=1) of the following reaction: Mn+Cl-n*mH2O (s) --> Mn+(aq)+nCl-(aq)+mH2O (l) for both CaCl_2*2H_2O & NH_4Cl using the table below. (The convention is that the standard enthalpy of the formation of ions in solution is referenced to 1 molal.)arrow_forward
- i need help with this questionarrow_forwardHey, The issue I am having here is calculating the partial pressure, if I use P=(n/V)*R*T then I get the partial pressure of H2 as 23.2 but according to the teacher it should be 11.585 so instead of just calculating the the total moles of H2 in the reaction should n be a molar fraction?arrow_forwardPLEASE help me with all parts of this problem. DOUBLE CHECK YOUR ANSWERS PREVIOUS TUTORS GOT IT WRONG.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY