
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
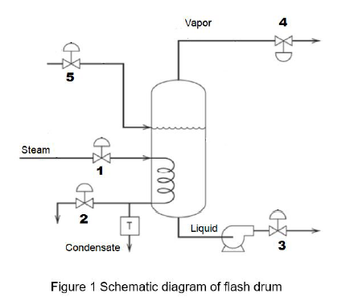
Figure 1 shows a schematic diagram of a flash drum. Steam in the steam coil is used to vaporize part of the liquid feed, and the liquid product is removed by the pump. There are 5 control valves installed to the flash drum.
Determine a suitable manipulated variable and a control valve (1-5) that can be used in the feedback control system to achieve the following control objectives:
i. The pressure of the vessel and the inlet steam flow rate
Please draw a complete P&ID of the feedback control loop (including transducer) for both control objectives.
Transcribed Image Text:Steam
5
D
2
Condensate
мее
Vapor
Liquid
Q
4
3
Figure 1 Schematic diagram of flash drum
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- show steos on how to reach part c) ans = 1.4mA/mAarrow_forwardProblem #1: The art of being a good chemical engineer is being able to quickly understand relationships between various phenomena. We oftentimes compare process variables (flow rate, temperature, pressure, etc.) to develop a mathematical understanding of a particular situation. Reactant concentration versus time, distillation pressure versus distillate (or bottoms) composition, and inlet flow rate versus outlet stream temperature of a heat exchanger are just a few of these. In gas chromatograph (GC), the GC converts a signal output (usually in mV) into a peak area. Calibration curves are made by testing the peak areas for different concentrations of a mixture and then fitting those areas to a linear expression. Using linear regression, develop the calibration curve for this dataset of various concentrations for monoethanolamine in toluene. Peak Area Conc, mg/L 0.05 0.03 5 0.13 7.5 0.25 10 0.33 12.5 0.35 15 0.44arrow_forwardConsider a perfectly mixed stirred tank heater with a single feed stream and a single product stream as shown in figure given below. Assuming that the tank is perfectly insulated. The flow rate (Fi) and temperature (Ti) of the inlet stream and the rate of heat added per unit time (Q) can vary with time. For steady state inlet and outlet flow rates of 100 L/min, a liquid volume is 500 L and inlet and outlet temperatures are 20 °C and 40 °C respectively. Find the steady-state heating rate. Density=1 kg/L, Cp=4184 J/kg.K F₁ T₁ 4 do Y 4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The