
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please do d, e, f I submitted a,b, c before thank you
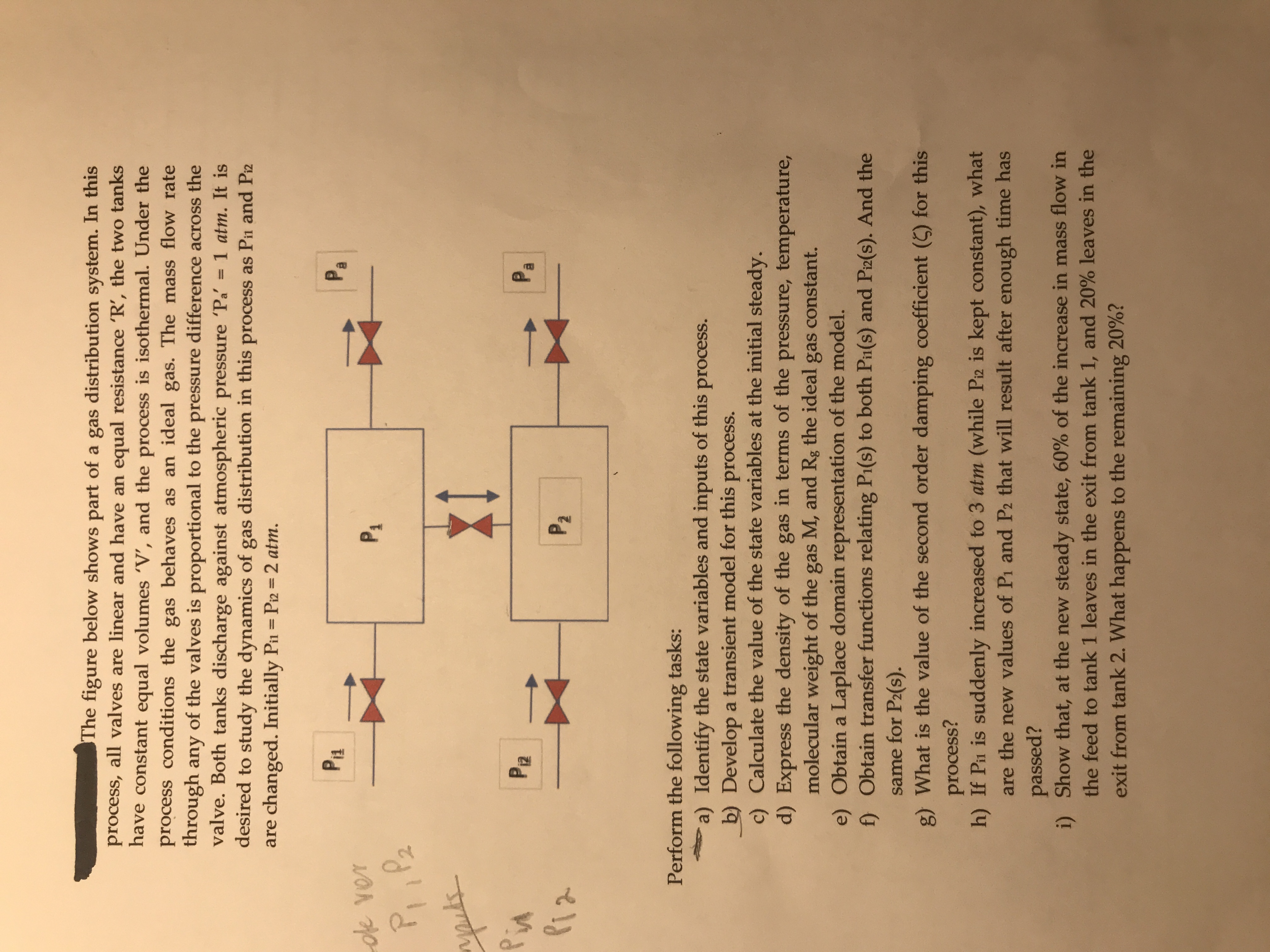
Transcribed Image Text:The figure below shows part of a gas distribution system. In this
process, all valves are linear and have an equal resistance 'R', the two tanks
have constant equal volumes 'V', and the process is isothermal. Under the
process conditions the gas behaves as an ideal gas. The mass flow rate
through any of the valves is proportional to the pressure difference across the
valve. Both tanks discharge against atmospheric pressure 'Pa
desired to study the dynamics of gas distribution in this process as Pin and Piz
are changed. Initially Pin = Pi2 = 2 atm.
1 atm. It is
%3D
P1
Pa
oe ver
P1
P, ,Pz
apuls
Pin
Piz
PR
Pa
P2
Perform the following tasks:
a) Identify the state variables and inputs of this process.
b) Develop a transient model for this process.
c) Calculate the value of the state variables at the initial steady.
d) Express the density of the gas in terms of the pressure, temperature,
molecular weight of the gas M, and Rg the ideal gas constant.
e) Obtain a Laplace domain representation of the model.
f) Obtain transfer functions relating Pi(s) to both Pi(s) and Piz(s). And the
same for P2(s).
g) What is the value of the second order damping coefficient (5) for this
process?
h) If Pin is suddenly increased to 3 atm (while Piz is kept constant), what
are the new values of Pi and P2 that will result after enough time has
passed?
i) Show that, at the new steady state, 60% of the increase in mass flow in
the feed to tank 1 leaves in the exit from tank 1, and 20% leaves in the
exit from tank 2. What happens to the remaining 20%?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 8 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- For a batch rectifier with appreciable column holdup, why do tray compositions change less rapidly than they do for a rectifier with negligible column holdup, and why is the separation improved?arrow_forwardcan you present your working in fullarrow_forwardIn the development of an operating policy (campaign) for batch distillation, what is done with intermediate (slop) cuts?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The