
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
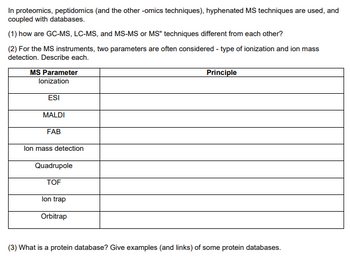
Transcribed Image Text:In proteomics, peptidomics (and the other -omics techniques), hyphenated MS techniques are used, and
coupled with databases.
(1) how are GC-MS, LC-MS, and MS-MS or MS" techniques different from each other?
(2) For the MS instruments, two parameters are often considered - type of ionization and ion mass
detection. Describe each.
MS Parameter
Principle
lonization
ESI
MALDI
FAB
lon mass detection
Quadrupole
TOF
lon trap
Orbitrap
(3) What is a protein database? Give examples (and links) of some protein databases.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
pls answer the last 3
TOF
ion trap
orbitrap
Solution
by Bartleby Expert
Follow-up Question
pls answer the next 3 pls
FAB
ion mass detection
quadrupole
Solution
by Bartleby Expert
Follow-up Question
number 2 pls
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
pls answer the last 3
TOF
ion trap
orbitrap
Solution
by Bartleby Expert
Follow-up Question
pls answer the next 3 pls
FAB
ion mass detection
quadrupole
Solution
by Bartleby Expert
Follow-up Question
number 2 pls
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the Ksp for uraninite (reaction 1) and rutherfordine (reaction 2). UO2 + 4 H+ ---U4+ + 2 H2O (reaction 1) UO2CO3 ---UO22+ + CO32- (reaction 2)arrow_forward5. The purpose of SDS in SDS-PAGE is (a) to selectively bind the target protein. (b) to maintain buffer pH in the gel. (c) to cause separation to be on the basis of molecular weight only. (d) to initiate polymerization of acrylamide to form a gel. (e) none of the above.arrow_forward5. Consider a standard C-18 reversed-phase column. Suppose you applied a globular protein to the column and measured the elution time. You then ran the column a second time using the same protein, except you heat-denatured the protein before applying it to the column. Assume that both the native and denatured proteins are soluble, and that they do not change their respective conformations during the column run. Which form should migrate through the column faster, the native form of the protein, or the denatured form of the protein? Explain briefly.arrow_forward
- Past paper questionarrow_forwardA sample of chromatin was partially digested by the enzyme staphy- lococcal nuclease. The DNA fragments from this digestion were purified and resolved on a polyacrylamide gel. A set of DNA restric- tion fragments was used as markers. Distances of migration are given below. From these data, estimate the nucleosome repeat dis- tance in the chromatin. Marker DNA Fragment Chromatin DNA Fragment Size (bp) d (cm) d (cm) 94 40 30.5 145 34.2 19.2 263 25.2 14.4 498 16.7 11.5 794 11.5arrow_forwardPrepare a report (formatting specifications below) outlining the 2020 COVID-19 drug authorization process a Canadian manufacturer of COVID-19 drugs and vaccines could use, as well as the necessary regulatory submission to maintain market authorization. The report should include, but is not limited to the following elements: Purpose/objective/overview of the temporary (interim) authorization pathway. Description of: key elements that were introduced in the temporary authorization pathway to increase authorization efficiency (describe at least 3) the regulatory submission requirements the same manufacturer must fulfill to maintain market authorization following expiration of the interim authorization. Detailed critical analysis of 2 new elements introduced in the temporary authorization pathway. You may select any 2 new elements found in the temporary authorization pathway. Your analysis should indicate the advantages of disadvantages of these two components. The critical analysis should…arrow_forward
- In order to improve the peptide separation by using a HPLC system, trifluoroacetic acid acts as mobile-phase modifier was added during the preparation of mobile phase. The preparation was performed by a postgraduate student as following:“2.851 g trifluoroacetic acid (MW: 114.02 g/mol) was made up to 500 cm3 in a graduated flask. To this solution, 50 cm3 of ethanol was added, and after mixing the mobile phase was placed in the solvent reservoir and pumping was commenced at 1.5 cm3 min-1.”Based on the given preparation procedure, identify THREE mistakes that were made.arrow_forwardSuh and Savizky studied the thermodynamics of human a - lactalbumin (HLA) unfolding in the absence (apo) or presence of various metal ions (Suh, J. J. and Savizky, R. M., Int. J. Biophys., 2011, 1(1), 1-6). You may assume that the protein starts out in the folded state and becomes denatured when it unfolds. According to the data below, which metal ion results in the greatest enthalpic stabilization of the folded state? AS (kJ/mol*K) 0.61 ± 0.07 0.55 ± 0.02 AH (kJ/mol) Experiment Аро-HLA MĘC1; + apo-HLA ZnCl, + apo-HLA CdCl, + apo-HLA CoCh + apo-HLA MnCl, + apo-HLA SICI, + apo-HLA CaCl + apo-HLA - apo-HLA KC1+ apo-HLA AG (KJ/mol) 184.92 ± 20.78 -5.29 ± 0.81 4.10 ± 0.17 3.62 0.13 174.05 ± 5.72 121.84 ± 6.02 0.38 ± 0.02 181.79 ± 11.56 6.06 = 0.46 0.57 ±0.04 184.69 ± 10.47 10.68 ± 0.44 0.56 ± 0.03 238.65 ± 15.22 14.10 ± 0.88 0.72 ± 0.05 15.08 ± 0.91 19.91 ± 1.33 223.41 ± 10.61 0.67 +0.03 232.16 ± 14.87 0.68 0.04 NaCl+a 162.17 ± 6.27 -4.19 + 0.18 0.54 + 0.02 154.02 ± 8.73 -3.58 ± 0.24 0.51…arrow_forwardMindTap - Cengage Learnir X G To analyze the reaction, firs x eploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1774598910&snapshot!... Bb DO: MLM HW 2.5- Blackbox Submit Answer References Use the References to access important values if needed for this question. A chunk of iron weighing 18.1 grams and originally at 97.77 °C is dropped into an insulated cup containing 80.4 grams of water at 23.75 °C. Assuming that all of the heat is transferred to the water, the final temperature of the water is mine Csp or T Final: Thermal Equilibrium in Metal + Water: This is group attempt 1 of 10 MacBook Air Autosaved at 9:15 PM Bb My Blackbo. °C. Q 1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY