
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:°F
budy
|||
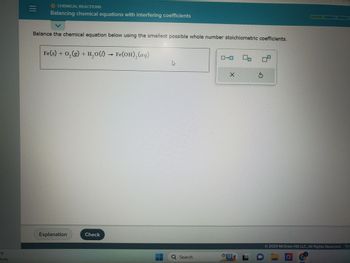
CHEMICAL REACTIONS
Balancing chemical equations with interfering coefficients
Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients.
Fe(s) + O₂(g) + H₂O(l) → Fe(OH)₂(aq)
Explanation
Check
4
Q Search
1-0
X
0₁
OL
TEENITTY
09
© 2023 McGraw Hill LLC. All Rights Reserved. Teri
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Want to make sure i did these correctly. Please help me verify. Show work if possible. Appreciate you.arrow_forwardBalance the following skeleton reaction, add up all the stoichiometric coefficients and choose the correct sum total. Remember if you wrote no coefficient it is assumed that there is a one there. Make sure any ones are in your sum total. N2(3) + H2(g) -----> NH3@) 12arrow_forwardWhat is the coefficient for water when the reaction is balanced with the smallest whole number ratios in acidic solution? Cr2072-(aq) + Mn²*(aq) --> Cr³*(aq) + MnO2(s) unbalanced O 5 O 1 O 2 O 4 « Previous Nextarrow_forward
- Use the precipitation interactive to select the solution that reacts with the Ba(NO,), solution to form a white solid. K,CO, KI O K,S Identify the precipitate that forms and the spectator ions that remain in the solution following this reaction. Answer Bank S (aq) Ва* (аq) BaS(s) BaCO, (s) KNO, (s) I(aq) NO, (aq) Bal, (s)arrow_forwardPls explained the whole steps with correct solution i will upvote you otherwise downvote remember downvotearrow_forwardBalance: CH2O + KMnO4 = CH2O2 + MnO2 under neutral conditionsarrow_forward
- Enter { Backspace Delete てレヨ PrtScr しレヨ F8 F7 FS DELL 4/23/2022 4:21 PM O 2022 McGraw Hill LLC. AI Rights Reserved. Terms of Use Privacy Center | Accessibility Check Explanation D-0 Write the net chemical equation for the production of iron from carbon, oxygen and iron(III) oxide. Be sure your equation is balanced. Fe,03(s)+3 Fe(s)+3 CO,(g) In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide: 2 C(s)+O,(g)→2CO(g) Blast ex pure iron from the iron(III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide: Iarrow_forwardQuestion 2 of 6 Submit Write the balanced NET ionic equation for the reaction when aqueous BaCl, and aqueous (NH,)2SO, are mixed in solution to form aqueous NH,Cl and solid BaSO4. 3C,2- Reset 1 2 3 4 5 6. 7 8 9 + (s) (1) (g) (aq) H Ba S CI • x H2O Tap here or pull up for additional resourcesarrow_forwardBalance the following equation for a reaction in acidic solution. Only Ht or H20 may be added. (enter your answer as the sum of the coefficients) P4(s) + NO3 (aq) → H3PO4(aq) + NO(g) Submit Answer Tries 0/99 This discussion is closed. Send Feedbackarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY