
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
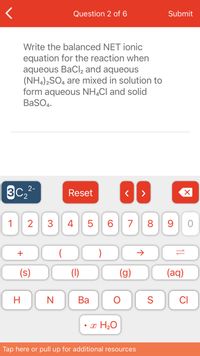
Transcribed Image Text:Question 2 of 6
Submit
Write the balanced NET ionic
equation for the reaction when
aqueous BaCl, and aqueous
(NH,)2SO, are mixed in solution to
form aqueous NH,Cl and solid
BaSO4.
3C,2-
Reset
1
2
3
4 5
6.
7
8 9
+
(s)
(1)
(g)
(aq)
H
Ba
S
CI
• x H2O
Tap here or pull up for additional resources
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- write a balanced chemical reaction for the reaction of vinegar, CH3COOH(aq) and baking soda, NaHCO3(s)arrow_forwardHow many grams of magnesium phosphate, Mg3(PO4)2, would you need if you were required to make 270.0 mL of a magnesium phosphate solution with a concentration of 0.170 mol L-1? Molar masses: Mg3(PO4)2 = 262.9 g mol-1 1 L = 1000 mL What is the concentration of PO43- ions in the 0.170 mol L-1 solution of magnesium phosphate?arrow_forwardComplete the balanced molecular chemical equation. If not reaction occurs, write NR Fe(NO3)(aq) + NaOH(aq)--arrow_forward
- Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of K 2S and Fe(NO 3) 2 are mixed.arrow_forwardWrite the balanced NET ionic equation for the reaction when aqueous BaCl₂ and aqueous (NH₄)₂SO₄ are mixed in solution to form aqueous NH₄Cl and solid BaSO₄.arrow_forwardWrite the balanced COMPLETE ionic equation for the reaction when Na:COs and AGNO3 are mixed in aqueous solution. If no reaction occurs, simply write only NR. 03- O D2+ N3+ 14+ 1 2 3 4 7 9 0 (s) (1) (g) ][(aq) Ag Na NR Reset x H¿Q Delete Co CO LO +arrow_forward
- Write the balanced NET ionic equation for the reaction when aqueous NiCl₂ and aqueous Na₂S are mixed in solution to form solid NiS and aqueous NaCl.arrow_forwardA solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO3)2(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 17.07 g PbCl2(s) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb(NO3)2(aq) solution.arrow_forwardWrite a balanced chemical equation for this combination reaction, having correctly identified the product as Ca(OH)2 (aq)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY