
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
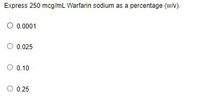
Transcribed Image Text:Express 250 mcg/mL Warfarin sodium as a percentage (wN).
O 0.0001
0.025
0.10
0.25
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- cloudy ||| O CHEMICAL REACTIONS Calculating molarity using solute moles Calculate the concentration in umol/L of the chemist's silver(I) nitrate solution. Round your answer to 3 significant digits. µ mol L A chemist prepares a solution of silver(I) nitrate (AgNO3) by measuring out 319. μmol of silver(I) nitrate into a 250. mL volumetric flask and filling the flask to the mark with water. Explanation Check x10 X Ś INTBcSzwZ0GGhrmlP4yCejeRhX9HIzqlzRTj2iaDBXTCRIHYWNO_-o? 10... Q Search 2 3/5 Jessica O 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility wwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwww E 00 Ararrow_forwardIf 0.42 mg calcium fluoride, CaF2 is used as above to add fuoride to the drinking water, doesCaFz add more fluoride than 0.42 g of NaF in the 250 mL glass of water? Justify your answer.arrow_forwardComplete the following table by calculating values for the two serial dilutionsarrow_forward
- How many mL of liquid mercury with a density of 13.6g/mL must you dispense to have 1.56 x 10-3 molarrow_forwardThe mass of weighing bottle +sulphamic acid=3.38g The mass of bottle empty= 0.97 g RMM of sulphamic = 2.41 g What’s the moles of sulphamic acid in 1000cm^3 of solution? (Molarity of sulphamic acid)arrow_forwardGiven the reaction: 2 Na(s) + Cl2(g) → 2 NaCI(s) Which statement is true? O The conversion factor for chlorine gas to sodium chloride is: 1 mol Cl2 is equivalent to 2 mol NaCI O The conversion factor for chlorine gas to sodium chloride is: 2 mol Cl is equivalent to 2 mol NaCl O The conversion factor for sodium metal to sodium chloride is: 1 mol Na is equivalent to 2 mol NaCl O The conversion factor for sodium metal to sodium chloride is: 2 mol Na is equivalent to 1 mol NaCIarrow_forward
- 5. The synthesis of aspirin (acetylsalicylic acid) is shown below. ملا OH OH salicylic acid ("SA”) ● + H3C CH3 acetic anhydride ("AA") H3C OH aspirin ("AS") a) If you start with 0.25 grams of salicylic acid and 2.0 mL of acetic anhydride (d=1.08 g/mL), how much aspirin can you theoretically synthesize? + CH3COOH Show two calculations for the theoretical yield of aspririn: 1 with 0.25 g SA and 1 with 2.0 mL of AA. Compare the theoretical amounts of aspirin from both calculations to determine the limiting reagent. Circle/label the limiting reagent and its theoretical yield of aspirinarrow_forwardWhen a Vitamin C (ascorbic acid; MM = 176.12 g mol-1) tablet is crushed, dissolved and titrated with 0.0377 M KIO3(aq) to a purple/blue endpoint (given by a starch indicator), the volume of KIO3 used is 40.01 mL. How many mg of Vitamin C were in the tablet? KIO3(aq) + 5 KI + 6 H+ → 3 I2(aq) + 3 H2O I2 (aq) + ascorbic acid → 2 I- + dehydroascorbic acidarrow_forwardA solution containing 78 g of NaNO3 in 70. g H2O at 50 ° C is cooled to 20 °C. Use the solubility data from the table below. Part A Solubility (g/100. g H2O) Substance 20 °C 50 °C How many grams of NaNO3 remain in solution at 20 °C? KCI 34 43 Express your answer in grams to two significant figures. NaNO3 88 110 ? C12 H22O11 (sugar) 204 260arrow_forward
- Toxic Cr(VI) can be precipitated from an aqueous solution by bubbling SO2 through the solution. How much SO2 is required to treat 3.00 x 108 L of 6.50x102 mM Cr(VI)? 2C:0+3s0, +4H* Cr, (SO4)3+2H,0 4/3arrow_forwardCalculate the mass of potassium hydrogen phthalate (KHP) molar mass=204.22 P) (₁ your answer has the correct number of significant digits. g KHP 0 x10 X g mol required to neutralize 20.00 mL of a 0.250 M NaOH solution. Be surearrow_forwardD D D D Δ ΔΔΔΙΔ to e quivalents 2 σκελί Convert 1.87 mol50, 2007 31. Eqarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY