
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please show mechanism
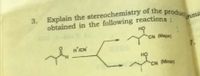
Transcribed Image Text:Explain the stereochemistry of the produc
3.
obtained in the following reactions:
Snsu
но
CN (Major)
H'ICN
7.
HỌ
CN (Minor)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Of the following, ________ is endothermic. Group of answer choices condensation all these processes are endothermic. fusion deposition crystallizationarrow_forwardData and Report Sheet - Plastics Name Define a Polymer. Date Choose one of the polymers you tested. 1) Draw a representation of the structure. I 2) Briefly discuss its history and an interesting mechanical property and thermal property. 25arrow_forwardRank the following elements by electron affinity, from most positive to most negative EA value. Iodine, Neon, Sulfer, Arsenic, Sodium.arrow_forward
- Please do all parts if possible, I dont need explanation just answer, Thank you: TRUE OR FALSE: a) Glasses are usually harder than plastics but softer than silicon carbide and boron carbide b) Any amorphous (lacking in long-range periodic order) materials, inorganic, organic, or metallic, formed by any techniques, exhibits glass transformation formation behavior are glasses c) In general, the viscosity is low at the melting position of glass, and high at room temperature. d) In general, if the viscosity is very high at the melting position of the corresponding crystalline phase which would form the melt, it is much easier to form glass for this melt. e) The glass fiber is possible to have a tensile strength even larger than that for iron or structural steel. Therefore, in the practical applications, we should be able to use glasses for the same purposes of the iron or structural steel.arrow_forwardClick and drag from the options below to complete these sentences. Answers may be used more than once. In a continuous fibre reinforced composite where the fibres are well aligned, the strength in the transverse direction will be the strength in the longitudinal direction. The stiffness of the composite in the transverse direction will be the stiffness in the longitudinal direction. greater than similar to less thanarrow_forwardwith the For a poly(hexamethylen-adipamide) polymerization degree of 200, write the structure and compute the exact and approximate molecular mass.arrow_forward
- View Help ✓ FALT Paragraph Search Heading 1 Thermometer Fractionating column Water Normal 1. Show derivation for the Clausius-Clapeyron equation. 2. Draw a phase diagram for molecular Iodine I2. 3. What is the name and (technique) shown below? Describe how this piece of equipment separates substances. Heater Condenser Distilling flask 1 Water Receiving flask No Spacing Adapter 29 I Heading 2 Styles Titlearrow_forwardIn Langmuir model for reversible adsorption, if the ratio between the density of occupied surface by protein ( 8.45 ) and the initial protein concentration were 0.5. If the affinity constant (K) were equal to 3; and the total number of surface sites were equal to 2. What is the initial protein concentration in the fluidarrow_forward4. An alloy has an elastic modulus of 115 GPa. For the alloy, plastic deformation begins at a stress of 275 MPa. (a) If a sample of cross-sectional area 325 mm? is obtained from the alloy, determine the maximum load that may be applied on the sample without plastic deformation. (b) The original sample length is specified as 115 mm, what is the maximum length to which the sample can be stretched without plastic deformation.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY