Explain the properties of Constitutional isomers ?
Isomerism-molecules with same molecular formula but different structural formula called as isomers and phenomenon is called as isomerism.
Constitutional isomers - isomers with same molecular formula but different connectivity (functional group) and also having different bonding pattern.
e.g. ethyl alcohol and dimethyl alcohol are constitutional isomers.
Also it can be classified for alkanes as:
1. Chain isomerism-in an alkane if long chain of carbon is present called as chain isomers.
e.g. n-pentane
CH3-CH2-CH2-CH2-CH3
2. Position isomerism-
On a specific position atoms or group of atoms are present called as position isomerism.
e.g.2-Methyl butane
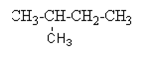
Answer of given question:
Properties of constitutional isomers:
- They have same number of atoms but structure (constitution) is different.
- They have different physical properties like melting and boiling point.
- They have same chemical properties.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images









