
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
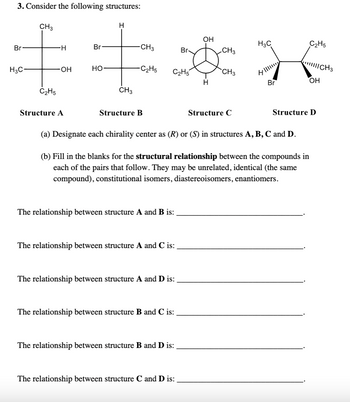
Transcribed Image Text:3. Consider the following structures:
Br
H3C-
CH 3
C₂H5
-H
-OH
Structure A
Br
HO-
H
CH3
-CH3
-C₂H5
Structure B
C₂H5
The relationship between structure A and B is:
The relationship between structure A and C is:
The relationship between structure A and D is:
The relationship between structure B and C is:
Br
The relationship between structure B and D is:
The relationship between structure C and D is:
OH
H
CH3
CH3
(a) Designate each chirality center as (R) or (S) in structures A, B, C and D.
(b) Fill in the blanks for the structural relationship between the compounds in
each of the pairs that follow. They may be unrelated, identical (the same
compound), constitutional isomers, diastereoisomers, enantiomers.
Structure C
H₂C
H
Br
4||||
C₂H5
||||||CH 3
OH
Structure D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwriting solutionarrow_forward3.54 THC is the active component in marijuana, and ethanol is the alcohol in alcoholic beverages. Explain why drug screenings are able to detect the presence of THC but not ethanol weeks after these substances have been introduced into the body. OH HO, ethanol tetrahydrocannabinol THCarrow_forwardDraw a skeletal ("line") structure of this molecule: HỌ CH, CH3 CH₂ CH₂-CH-CH3 Click and drag to start drawing a structure. C C Carrow_forward
- please answer all questionsarrow_forward9. Give the compound name, functional group name, and organic family name in each Ose. Classify alcohols as primary (1°), secondary (2 ), or tertiary (3"). Organic family Functional Formula Compound name group name CH3-CHz-CH-CH2-OH 1. CH3 CH,-CH,-0-CH,-CH,-CH, || CH,-CH, C-H CH CH,-C-CH-CH,-CH, CH-CH-CH- CH- COOHarrow_forwardWhen CH3COOH(ℓ) is dissolved in water, what covalent bonds break?arrow_forward
- ► < (b) H₂C B B W Chapter... 135 Provide the systematic (IUPAC) name for each of the following saturated hydrocarbons. (a) H₂ H₂C- (c) H₂C H₂C H₂ H₂ -C H₂C H3C CH₂ H₂C H₂C CH₂ H₂ HC CH3 CH₂ H₂ H₂ CH₂C H₂ CH₂ CH₂ H₂C CH₂ CH G + webassign.net C S E MacBook Air Garrow_forwardwhat class is this compound?arrow_forwardAnswer the following questions: 1. Name the following compounds according to IUPAC rules. A, ( C, ( CI CI CI B, ( D, ( F Br CI )arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY