
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Profiles
Tab
Window
Help
4 Recent - Google Drive
G Olympic cyclists fi
t- Goo x
E Exp. 6 Analysis & Discus: X
tempt=323292&cmid%3D1445098
ax
A Sa
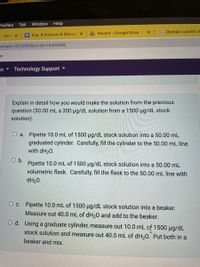
Technology Support
Explain in detail how you would make the solution from the previous
question (50.00 mL a 300 µg/dL solution from a 1500 ug/dL stock
solution).
O a. Pipette 10.0 mL of 1500 µg/dL stock solution into a 50.00 mL
graduated cylinder. Carefully, fill the cylinder to the 50.00 mL line
with dH20.
O b.
Pipette 10.0 mL of 1500 ug/dL stock solution into a 50.00 mL
volumetric flask. Carefully, fill the flask to the 50.00 mL line with
dH20.
O c. Pipette 10.0 mL of 1500 µg/dL stock solution into a beaker.
Measure out 40.0 mL of dH20 and add to the beaker.
d. Using a graduate cylinder, measure out 10.0 mL of 1500 µg/dL
stock solution and measure out 40.0 mL of dH,0. Put both in a
beaker and mix.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- SHOW YOUR WORK! Use conversion factors to determine the answers to the Following questions. Do not forget sig figs & units. #47arrow_forwardCalculate the percent mass per volume, % (m/v), of a dextrose solution containing 7.00 g of dextrose in 3.00 × 10² mL of solution. Note that mass is not technically the same as weight, but the abbreviation % (w/v) is often used interchangeably with % (m/v). mass/volume %: % (m/v)arrow_forwardPlease don't provide handwritten solution...arrow_forward
- Are these correct? The top page of the second paper is the second problem.arrow_forward5. The mineral analysis below was reported for a water sample. Calcium 90 Chloride 120 Magnesium 30 Sulfate 170 Iron (Fe**) 5 Bicarbonate 165 Sodium 10 pH 7.5 units Potassium 6. Note: All reported as "mg/L as the ion" unless stated otherwise. Draw a bar chart that includes all the ions listed above. (Remember to convert to appropriate units) b. What is the total hardness of the water using ALL multivalent cations in mg/L as а. CACO3? c. What is the total hardness of the water using the predominant polyvalent cations in mg/L as CaCO3? d. What is the percent error in using only the predominant polyvalent cations? e. What is the carbonate hardness of the water in mg/L as CaCO3? f. What is the non-carbonate hardness of the water in mg/L as CACO3?arrow_forwardBiological studies have found that aqueous solutions containing as little as 28 µg of allicin per milliliter inhibit bacterial growth. Express this amount as the number of moles of allicin per liter of solution. \(28~\text{ug/mL}=\) \(text{mol/L}\) 20 F3 II F8 F4 F5 F6 F7 F9 F10 F11 F12 & %24 4. 3 5 7 9 { } P E T Y U %3D * CO R %23arrow_forward
- number 15 (POST) please answer A&B ! thank you!arrow_forwardDetermine a procedure to accurately create a 1.5•10^-6 mg Fe/mL solution using a 0.0500 mg Fe/mL standard solution and only having access to 100.0 mL volumetric flasks and 1.00, 2.00, 3.00, 4.00, 5.00 and 10.00 mL volumetric pipets. (You may have it make more than one solution by dilution to accomplish this)arrow_forwardCan someone help me with number 7 please ☺️?arrow_forward
- Interpolate the solubility of NH4Cl at 80°C. using the graph. Display your work on the graph.arrow_forwardYou have a 20% stock of NaCl, a 30% stock of K2HPO4, 2.5 M NH4Cl and 10 mg/ml stock of ampicillin. a. How many grams of NaCl would you need to weigh out to make up 150 ml of a solution?b. You will be preparing 300 ml of media. You need to dilute this 20% stock of NaCl, the 30% stock of K2HPO4, the 2.5 M NH4 how much stocks you need to add. Fill in the table below. Stock Calculate Final Concentration needed ml added20% NaCl 1.5% ? 30% K2HPO4 2% ? 2.5 M NH4 Cl 0.1M ? 10 mg/ml ampicillin water 0.05 mg/ml ? water To 300 ml ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY