
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
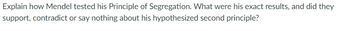
Transcribed Image Text:Explain how Mendel tested his Principle of Segregation. What were his exact results, and did they
support, contradict or say nothing about his hypothesized second principle?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the molecules: CH2=CH-CH=CH-CH=CH-CH=CH-CH=CH2. Let’s assume that the 10 electrons that make up the double bonds can exist everywhere along the carbon chains. The electrons can then be considered as particles in a box; the ends of the molecule correspond to the boundaries of the box with a finite or zero potential energy inside. In this “molecular box”, 2 electrons can occupy an energy level. What are quantum states that the electrons from this molecule can occupy in the ground state? Note that the length of a C-C bond is about 1.54A and the length of a C=C bond is 1.34A to allow you to estimate the length of the “molecular box”arrow_forwardSchrodinger and de Broglie suggested a ‘Wave—particle duality" for small particles—that is, if electromagnetic radiation showed some particle-like properties, then perhaps small punicles might exhibit same wave-like properties. Explain. How does the wave mechanical picture of the atom fundamentally differ from the Bohr model? How do wave mechanical arbitals differ from Bohr’s orbits? What does it mean to say that an orbital represents a probability map for an electron?arrow_forwardWhat are the units of the answer, 4426?arrow_forward
- For 1 I have A being B, B. No, C. Yesarrow_forwardScheme C Scheme B Scheme A 1) Cl2 (xS) 2) NaOH (xs) 3) H3O* 4) SOCI2 5) 1) Cl2 (xS) 2) N2OH (xs) 3) H. 1) Cl2 (xS) 2) NAOH (xs) 3) HO (xs) Br O watigin ansers Mutigle answers are accepted for this question Sled one moe rs and smit. For keyboard navigation SHOW MO Scheme :ö:arrow_forwardWhat is Salkowski Test test for? What is the positive result for this test? What is the principle behind this test?arrow_forward
- Explain why it makes sense that most energies of formation on the data sheet are almost all negative amounts. (doesn't need to be in depth, i just need the answer quick if you respond after twenty minutes then reject it)arrow_forwardHello, Can you help me with this correcting me this questions below. Here I leave the comments to correct: In 3b, you didn't convert the Ea's to J/mol (also, why "45" and "76"? I don't see where those numbers came from). Also, the 298 should be in the denominator. It should end up that it's roughly 10^30 times faster. In 3c, NO. Try a higher temperature with the same Ea you used in 3b. The k should go down at a higher temperature. In 3d, NO. Mention that H2 is a product, and that this is an example of product inhibition.arrow_forwardSuppose you are standing at the exact center of a park surrounded by a circular road. An ambulance drives completely around this road, with siren blaring. How does the pitch of the siren change as it circles around you?arrow_forward
- When a hot block is placed next to a cold block the two blocks end up at the same temperature. Explain how both this happens (what molecular motion is happening and the direction and flow of energy) and why this happens (what is driving this and why it will not reverse) *****talk about quantaarrow_forwardShow that Q (ame) V 1 Q' ΤΟarrow_forwardA. The text shows that: ∂U/∂T)V = ∂U/∂V)P. Can you show that: ∂H/∂T)V = ∂H/∂T)P ? Hint: start with: ∂H = ∂H/∂T)P ∂T +... B. Starting with: ∂H/∂P)T = ∂(U+PV)/ ∂P)T , can you show that ∂U/∂P)T = ∂H/∂P)T ? Hint: you will need to use the perfect gas law.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning