
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Balance the following equations(b,c,d and e)
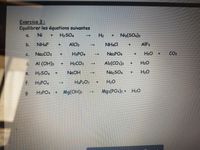
Transcribed Image Text:Exercice 3:
Equilibrer les équations suivantes
Ni
H2SO,
H2
Niz(SO.)3
a.
b.
NH&F
AICI3
NH&CI
AIF3
C.
NazCO3
H3PO4
NasPO4
+ H2O
CO2
d. Al (OH)3
H2CO3
Al2(CO3)3
H2O
H2SO4 +
NaOH
NazSO4
H20
->
e.
f. H3PO4
HAP2O7
H2O
H3PO4
Mg(OH)2
Mg3(PO4)2 + H20
9.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation (don't give Handwritten answerarrow_forwardBalance these chemical equations. (Use the lowest possible whole number coefficients.) (a) N2H4 + O2 → H2O2 + N2(b) Na2SO4 + C → Na2S + CO(c) Sr + H2O → Sr(OH)2 + H2(d) NH3 + O2 → NO2 + H2O(e) Fe(OH)2 + O2 + H2O → Fe(OH)3 (f) P2H4 → PH3 + P4arrow_forwardBalance the following chemical equations (type corresponding coefficients (1, 2, 3, ...) in blanks). COCI2 + H2O CO2 + HCIarrow_forward
- Identify the type of equation represented by the reaction below. C3H5(NO3)3 → CO2 + N2 + H20 O2 O Decomposition O Double Replacement O Single Replacement Synthesis @ # $ & backsp 2 4. e r y C V b ST 10arrow_forwardBalance these chemical equations. (Use the lowest possible whole number coefficients.) (a) S8 + O2 → SO3 chemPad Help (b) NaNO3 → NaNO2 + O2 chemPad Help (c) C4H10 + O2 → CO2 + H2O chemPad Help (d) AgCl2 + H2 → Ag + HCl chemPad Help (e) Ga + H2SO4 → Ga2(SO4)3 + H2 chemPad Help (f) N2H4 + O2 → H2O2 + N2 chemPad Helparrow_forwardWhich of the following particle models represents the balanced chemical equation? = S O = 0 + 00 SCroll Daum 88arrow_forward
- Adipic acid is used in the production of nylon, so it is manufactured in large quantities. The most common method for the preparation of adipic acid is the reaction of cylcohexane with oxygen. Balance the skeleton equation below. (Use the lowest possible whole number coefficients.)arrow_forwardBalance the reactionsarrow_forwardProvide examples, by using the only elements: cesium , beryllium , bromine and fluorine to design 6 equations eachshowing the following reactions, then balance each of them:a) Synthetic reaction eg. (A+ B= AB) substitute the letters with the selectedelements.b) Decomposition reaction eg. decomposition of CaCO3 (AB= A+B)c) Single displacement reaction eg. (A+BC= AC+B)d) Double displacement reaction eg. (AB +CD= AD + CB)e) Neutralization reactionf) Oxidation reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY