
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
How find X6
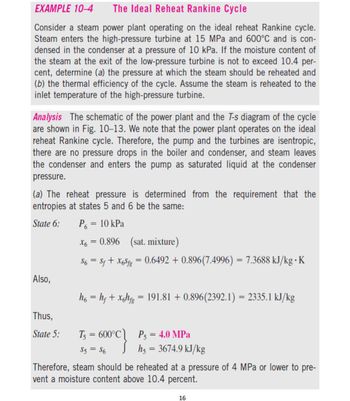
Transcribed Image Text:EXAMPLE 10-4
The Ideal Reheat Rankine Cycle
Consider a steam power plant operating on the ideal reheat Rankine cycle.
Steam enters the high-pressure turbine at 15 MPa and 600°C and is con-
densed in the condenser at a pressure of 10 kPa. If the moisture content of
the steam at the exit of the low-pressure turbine is not to exceed 10.4 per-
cent, determine (a) the pressure at which the steam should be reheated and
(b) the thermal efficiency of the cycle. Assume the steam is reheated to the
inlet temperature of the high-pressure turbine.
Analysis The schematic of the power plant and the T-s diagram of the cycle
are shown in Fig. 10-13. We note that the power plant operates on the ideal
reheat Rankine cycle. Therefore, the pump and the turbines are isentropic,
there are no pressure drops in the boiler and condenser, and steam leaves
the condenser and enters the pump as saturated liquid at the condenser
pressure.
(a) The reheat pressure is determined from the requirement that the
entropies at states 5 and 6 be the same:
State 6:
P₁ = 10 kPa
Also,
Thus,
State 5:
X6
0.896 (sat. mixture)
$6 = $f + X6Sfg = 0.6492 +0.896(7.4996) = 7.3688 kJ/kg K
=
h6 = hƒ + xghfg = 191.81 +0.896(2392.1) = 2335.1 kJ/kg
T₁ = 600°C P5 = 4.0 MPa
S5 = $6
Jh5 = 3674.9 kJ/kg
Therefore, steam should be reheated at a pressure of 4 MPa or lower to pre-
vent a moisture content above 10.4 percent.
16
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- A distillation column is used to separate 500 kg /h of a mixture containing 50 wt% benzene (C6H6) in toluene(C7H8), to give top product of 88 % mole benzene and 75% mole toluene waste (at bottom).1. Calculate the mole flow rate, mole fraction and mole percentage of each stream in the (F, D & W) aroundthe column? All results should be tabulated in the Table below.2. Calculate the heat removed by condenser? Given the following data;The enthalpy of feed stream is = - 900.00 kJ/kgmolThe enthalpy of distillate is = 800.00 kJ/kgmolThe enthalpy of waste is = 3000.00 kJ/kg molThe heat added by boiler is = 4500.00 kJ /hGiven:The molar mass of Carbon is 12The molar mass of Hydrogen is 1arrow_forwardWhat is the correct IUPAC name of the following compound? No capitalization, no italization, no spaces.arrow_forwardAnswer all the following, INCOMPLETE = REPORT. Please based your formula from the given (see attached)arrow_forward
- (#), = V - T(), av Derive the relationshiparrow_forwardHi! Can someone answer Learning task 4, no. 2? Thanks!arrow_forwardCanvas XC 2 X CO Summer 2024 Home Discussions Help Center The best grade will count as your homework grade. hw_Condensation al Ch E ndar box D Grades People Syllabus Quizzes Modules Collaborations Cisco Webex Library Resources Chat Score: 0/6 Answered: 0/1 12 Question 1 Microsoft OneDrive Class Notebook istory Microsoft Teams classes 凹 Studio Help K Microsoft Teams meetings University Syllabus Office 365 Adobe Creative Cloud Bulldog Bundle Digital Materials Credentials Steam is used on the shell-side of a vertical shell/tube exchanger to heat water. Given the following: Do = 0.75,in There are 100 tubes 14 ft long Condensing temperature 280 F and Heat of Vaporization = 1050 btu/lb The tube wall temperature can be assumed to be 195 F What is the condensate film temperature, F? 216.25 Also given: cond viscosity at 216.25 0.68077625 lb/ft-h cond k at 216.25-0.394 btu/ft-h-F cond cp at 216.25 -1.0 btu/lb-F cond density at 216.25 = 58.2 lb/ft3 What is the condensate prandtl number, Pr?…arrow_forward
- ot pt pt 1 pt 1 pt 1 pt FI 1 pt 1 pt 1 pt 2 An organic acid has pK₂ = 4.89. What is its K₂ value? a K₁₂ = Submit Answer F2 W 3 F3 E O: Q Search F4 Try Another Version 4 O R % 5 ro Activity.do?locator-assignment-take 3 item attempts remaining ASUS ZenBook F/ & C 7 0 H U [References] 0 FS 9 K с ITSarrow_forwardHow many Oxygen atoms would be produced from 18.75g Al(NO3)3? digit_______ (format of answer: 1.23x10^23) unit ________ Blank 1: Blank 2:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The