
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please explain as well. Thanks!
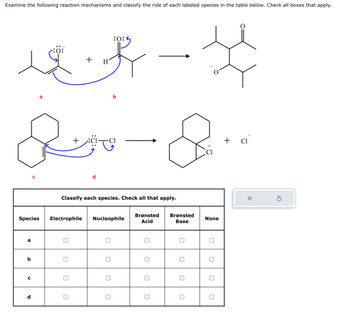
Transcribed Image Text:Examine the following reaction mechanisms and classify the role of each labeled species in the table below. Check all boxes that apply.
+ H
if - H
&-&
+
a
b
Species Electrophile Nucleophile
с
d
d
:0:6
Classify each species. Check all that apply.
Brønsted Brønsted
Acid
Base
0
0
None
+ Cl
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PLEASE SHOW YOUR WORK (THIS IS NOT A GRADED ASSIGNMENT)arrow_forwardNext E Contents Notes 3 A Grades 3 Riley Slusser Chemistry: Q4: 2022-23 | Bala 0³ 1 - 3.1H+1H → On+ On 2 O 4Be O ₂2H 2 He O 0 2 1Harrow_forwardWhat is/are the reagent(s) and conditions for the following reaction? 10 H Problem viewing the image. Click Preview Here Q Search H- 10. H. by 19 Il app.honorarrow_forward
- Which molecule is a complete organic molecule (i.e. all atoms are shown correctly)? A H. c=C–C= C–H H C H. C=C-C-C-H | H. H. C H. C- D H. нн . C С —С—Н C=C–C- C-H H H. ОН Н А I-0-I エーO I-Ú-I I-U-I I-U-I エー○arrow_forwardSee image belowarrow_forwardQ5. As part of the aspirin synthesis lab, the orgo students also had to perform the following calculation to demonstrate their knowledge. Are you able to help them work this out?Saponification is a process in which soap is produced from the chemical reaction between animal fat (triglycerides) and a strong base such as NaOH. An example of such a balanced chemical reaction is shown here:C57H110O6 + 3NaOH à C3H5(OH)3 + 3C18H35O2NaIf, during the saponification reaction, 228.5 g of C57H110O6 is mixed with 211.7 g of NaOH and 180 g of soap is produced: a. Calculate the theoretical yield of soap (in grams), C18H35O2Na, and indicate which species is the limiting reactant. Provide your answer to 2 decimal places. b. Calculate the percent yield for this reaction. Provide your answer to 1 decimal place. Show ALL steps and equations involved in your calculations. Remember to label all steps clearly and use appropriate unitsarrow_forward
- Write a paragraph explaining the meaning of the phrase "Its dose that makes the poison". Supoort your answer with examples of your own.arrow_forwardWhy are ducks waterproof? It’s because they produce copious amounts of oils from their uropygial glands and spread it across their feathers. In this exercise, we’ll be investigating the molecular structure of one of these preen oils to determine how it keeps ducks dry. Q.5 - Preen oil is actually a complicated mixture of many different organic compounds, such as the structure seen previously.. Ornithologists have determined that birds often use preen oil compounds for scent recognition. Below, several different chemicals isolated from preen oil are shown, along with their vapor pressures at room temperature. p-cymene has the highest vapor pressure, meaning it is the most easily evaporated compound of the three listed. Explain why p-cymene has a higher vapor pressure at room temperature compared to the other compounds. Make sure to explain what holds the p-cymene in the sample. (Image attached)arrow_forwardArrange these substance according to how many drops we could add to a penny before the "bubble" popped, FEW DROPS (left-most) to MANY DROPS (right-most). The description labels on the images are small, so use the following legend to identify the materials: A: vegetable oil B: motor oil C: dish soap D: syrup E: honey A CITE EVIDENCE from your Learning Experience and provide REASONING for why you arranged these substances in this order.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY