Question
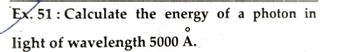
Transcribed Image Text:Ex. 51: Calculate the energy of a photon in
light of wavelength 5000 A.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 3.4 The threshold frequency for photoelectric emission in Copper is 1.1× 1015 Hz. Find the maximum kinetic energy of the photoelectrons emitted when light of frequency 1.5×1015 Hz is directed on a Copper surface.arrow_forwardWhat is the wavelength of the photon with energy E = 5.4 × 10-15 J. Use the unit of nm for the wavelengtharrow_forwardA log in the fire is glowing red (λ = 629 nm). What is the temperature of the log, in kelvin?arrow_forward
- The photoelectric equation for the kinetic energy of a photoelectron is, following Einstein, E ≤hf − W, where h is Planck’s constant, f is the frequency of the light, and W is the work-function.Sodium has W = 3.2×10−19 J. When sodium is illuminated by monochromatic light of a particularfrequency, electrons are emitted with speeds up to 8 × 105 m s−1.a) Calculate the wavelength of the light.b) Calculate the stopping potential.arrow_forwardBarium has a work function of 2.48 eV. 1. a) What is the maximum kinetic energy of electrons if the metal is illuminated by UV light of wavelength 365 nm? 2. b) What is their speed?arrow_forwardA) In what part of the EM spectrum would a photon of energy 4.9 ✕ 10−17 J be found? microwave infrared visible ultraviolet B) What is its energy in electronvolts?arrow_forward
- For a certain metal, the threshold wavelength for the photoelectric effect is 719.0 nm. What is the maximum velocity (in m/s) for ejected electrons when light with wavelength 441.0 nm shines on the metal?arrow_forwardThe photoelectric threshold wavelength of a tungsten surface is given as 256 nm. Calculate the maximum kinetic energy of electrons ejected by ultraviolet radiation with a frequency of 1.47 x 1015 Hz from the tungsten surface. (Express your answer in terms of electron volts.)arrow_forwardAn excited electron drops to its ground state, emitting a photon of frequency f = 5.10×1015 Hz. What is the energy of this photon? Enter the numerical value in units of eV.arrow_forward
- QUESTION 7 A metal having a work function of 2.8 eV is illuminated with monochromatic light whose photon energy is 3.9 eV. What is the threshold frequency for photoelectron production? (h-6.626 x 10-34 J-s-4.141 x 10-15, eV s, 1 eV 1.60 x 10-19 J CA 8.5 x 10¹4 Hz OB. OC 6.8 x 10¹4 Hz 7.6 × 10¹4 Hz 2.7 x 10¹4 Hzarrow_forwardA 610 keV gamma ray is incident on an electron at rest. If the scattered photon has a scattering angle of 112° with respect to the direction of the incident photon, determine the following. (a) Energy (in keV) of the scattered photon 2.094 How can you determine the wavelength and hence energy of the scattered photon? keV (b) Kinetic energy (in keV) of the scattered electron 40.2 How is the energy of the scattered electron related to the energy of the incident and scattered photons? ke (c) Recoil angle (in degrees) of the scattered electron 112 x Is momentum conserved during this process? How can you determine the momentum of the scattered photon and electron?°arrow_forwardAn X-ray photon with a wavelength of 0.999 nmnm strikes a surface. The emitted electron has a kinetic energy of 990 eV. What is the binding energy of the electron in kJ/molkJ/mol? [Note that KEKE = 12mv212mv2 and 1 electron volt (eVeV) = 1.602×10−19J1.602×10−19J.] Express your answer using three significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios