
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
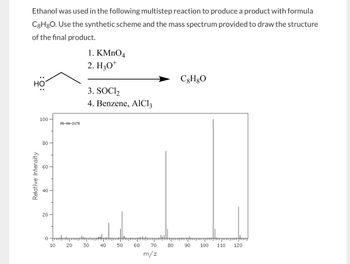
Transcribed Image Text:Ethanol was used in the following multistep reaction to produce a product with formula
C8H8O. Use the synthetic scheme and the mass spectrum provided to draw the structure
of the final product.
HO
1. KMnO4
2. H3O+
3. SOCI₂
4. Benzene, AlCl3
C8H8O
100
MS-NW-2178
80
66
60
Relative Intensity
40
20
20
0
10
20
30
8-
50
550
10
40
60
70
80
-8
90
100
110
120
m/z
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- USING ARROWS TO DENOTE ELECTRON FLOW- PROVIDE a detailed reaction mechanism for the reaction scheme belowarrow_forwardStarting materials Br Reagents OH 1 H3C OH 2 Br 6 Br 7 OH OH TOH OH 8 5 H a Mg / dry ether e Aqueous H2SO4 at reflux i KMnO4 / H3O+ b 1. CO2 2. acidic workup of Conc. HCI or HCI (gas) j Na2CrO4 / aqueous H2SO4 C NaCN/THF or DMF g PBr3 K 1. BH3/THF 2. H₂O2/aq. NaOH d NaCN/dil. aqueous H2SO4 h KOH alcohol |arrow_forwardTMS is usually used in NMR as a standard because it is soluble in a variety of solvents. It is, however, insoluble in D20. What is used as a standard in D₂0?@GMU 2020 O CDC13 O Acetone-d6 {CD3(C=O)CD3} O DSS {(CH3)3Si(CH2)3SO3¯Na*} O DMSO-d6 {CD3(S=O)CD3)arrow_forward
- I want to explain what happened in this reactionarrow_forwardD. Provide a reasonable mechanism for the following reaction. -CC13 1. NaOH, H₂O 2.H₂O* ooi OHarrow_forwardO 3G lI.. CHEM 310 and 312 Homework T... pi What are the products of the following reaction? Would you expect them to have higher or lower Amax than starting material? Justify your answer. NaOEt, EIOH Heat H2 Pd/Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY