
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
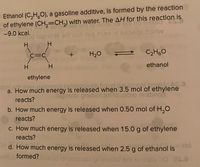
Transcribed Image Text:Ethanol (C,H,0), a gasoline additive, is formed by the reaction
of ethylene (CH,=CH,) with water. The AH for this reaction is
-9.0 kcal.
H
C=C
H20
C2H60
ethanol
ethylene
a. How much energy is released when 3.5 mol of ethylene
reacts?
b. How much energy is released when 0.50 mol of H,O
reacts?
c. How much energy is released when 15.0 g of ethylene
reacts?
d. How much energy is released when 2.5 g of ethanol is
HA
eritobformed?hb nemesie priwollot ort to bee od 20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the generic chemical equationaA+bBcC+dD (where a, b, c, and d represent coefficients for the chemicals A, B, C, and D, respectively). a. How many possible values are there for “c”? Explain your answer. b. How many possible values are there for “c/d”? Explain your answer.arrow_forward. A(n) _______ speeds up a reaction without being consumed.arrow_forwardWhy is it important to give the states of the reactants and products when giving an equation for H?arrow_forward
- A lead ore, galena, consisting mainly of lead(II) sulfide, is the principal source of lead. To obtain the lead, the ore is first heated in the air to form lead oxide. PbS(s)+32 O2(g)PbO(s)+SO2(g)H=415.4kJ The oxide is then reduced to metal with carbon. PbO(s)+C(s)Pb(s)+CO(g)H=+108.5kJCalculate H for the reaction of one mole of lead(II) sulfide with oxygen and carbon, forming lead, sulfur dioxide, and carbon monoxide.arrow_forwardUse Table 8.3 to obtain AHO for the following thermochemical equations: (a) Mg(OH)2(s)+2NH4+(aq)Mg2+(aq)+2NH3(g)+2H2O(l) (b) PbO(s)+C(s)CO(g)+Pb(s) (c) Mn(s)+4 H+(aq)+SO42 Mn2+(aq)+SO2(g)+2H2O(l)arrow_forwardHydrogen chloride gas dissolves in water to form hydrochloric acid (an ionic solution). HCl(g)H2OH+(aq)+Cl(aq) Find H for the above reaction. The data are given in Table 6.2.arrow_forward
- 9.89 A sample of gas is 80.0% CH4 and 20.0% C2H6 by mass. What is the heat from the combustion of 1.00 g of this mixture? Assume the products are CO2 (g) and H2O (l).arrow_forwardExplain why each of the following chemical equations is not a correct formation reaction: 4Al( s )+3 O 2 ( g )2 Al 2 O 3 ( s ) N 2 ( g )+ 3 2 H 2 ( g ) NH 3 ( g ) 2Na( s )+O( g ) Na 2 O( s )arrow_forward9.75 Explain why each of the following chemical equations is not a correct formation reaction. (a) 4Al(s)+3O2(g)2Al2O3(s) (b) N2(g)+32H2(g)NH3(g) (c) 2Na(s)+O(g)Na2O(s)arrow_forward
- The equation for the combustion of 2 mol of butane can be written 2C4H10(g)+O2(g)8CO2(g)+10H2O(g);HO Which of the following produces the least heat? a Burning 1 mol of butane. b Reacting 1 mol of oxygen with excess butane. c Burning enough butane to produce 1 mol of carbon dioxide. d Burning enough butane to produce 1 mol of water. e All of the above reactions (a, b, c, and d) produce the same amount of heat.arrow_forwardHow much heat is released when a mixture containing 10.0 g NH3 and 20.0 g O2 reacts by the following equation? 4NH3(g)+5O2(g)4NO(g)+6H2O(g);H=906kJarrow_forwardHypothetical elements A2 and B2 react according to the following equation, forming the compound AB. A2(aq)+B2(aq)2AB(aq);H=+271kJ/mol If solutions A2(aq) and B2(aq), starting at the same temperature, are mixed in a coffee-cup calorimeter, the reaction that occurs is a exothermic, and the temperature of the resulting solution rises. b endothermic, and the temperature of the resulting solution rises. c endothermic, and the temperature of the resulting solution falls. d exothermic, and the temperature of the resulting solution falls. e exothermic or endothermic, depending on the original and final temperatures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning