
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
5.
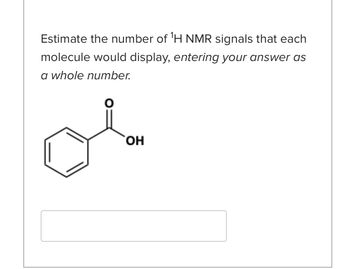
Transcribed Image Text:Estimate the number of ¹H NMR signals that each
molecule would display, entering your answer as
a whole number.
ملی
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- As indicated in step 2 above, flour contans starch (complex sugar): 1) 1.00 cup of flour contains 68% starch. How many grams of starch is this? ( 1 cup = 120 g) 2) Recipe calls for 3.25 cups of flour. How many grams of starch are in the bread?arrow_forwardSuppose 1.1780g CuCl, and 2.2773g of Na,PO, were reacted as in this experiment. What is the percentage yield of Cu,(PO,), if 0.9856g of Cu, (PO,), was isolated? (Use 380.12g/mol for Na,PO, and 170.48g/mol for CuCl, and 434.60g/mol for Cu,(P0,),) Be sure to check for the limiting reactant.arrow_forward10.4 mL of ethanol is allowed to undergo combustion with an excess of gaseous oxygen to produce carbon dioxide and water vapor. What mass of carbon dioxide will be produced from this combustion? The density of ethanol (C2H6O) is 0.789 g/mL. (3 sf)arrow_forward
- 3.arrow_forward6. In one experiment, a mixture of 0.250 mol of methane gas was burned in 1.25 mol of ogen sina sealed container. Find the limiting reactant and calculate the theoretical yield of water produced. 2.34 g of water was actually produced in the experiment, what is the percentage yield? (9) CH,(g) + 02(9) Co,(g) + H20G) Reply... :arrow_forwardCalculate the protein count of 100 g of a food, with a total amount of proteinof 20 g and the following composition of amino acids for each g of protein: 200 mg oflysine, 125 mg valine, 182 mg glycine, 290 mg proline, and 43 mg leucine. TwoWhole eggs (100 g) contain 12.8 g of protein and 86 mg of leucine, 70 mg of lysine,66 mg of valine for every g of protein.arrow_forward
- (5.5, 5.7 & 5.8) Classify the following reaction. Ba(NO3)2(aq) + K₂SO4(aq) --> O acid-base reaction O gas-forming reaction O decomposition reaction O precipitation reactionarrow_forwardA 19.51 g sample of impure methylamine, which contains 72.58% (by mass) of CH;NH: , is reacted with 30.81 g of pure oxygen gas: 2.1 4CH, NH,(2) + 90,(8) - 4C0,(8) + 10H,0(?) + 2N;(8) 2.1.1 What is the percentage yield of this reaction if 5.54 g of nitrogen gas is collected? 2.1.2 In another experiment, this impure methylamine was used as follows: • An unknown mass of the impure compound is dissolved in enough water to make 500.0 m of solution. • 20 ml of this solution was transferred by pipette to a clean 250 ml volumetric flask and made up to the mark. • The molarity of the CH;NH; in the final solution was determined to be 0.103 M. Determine the mass of CH;NH; present in the original amount of impure compound used make this solution.arrow_forward8.8arrow_forward
- 6. 7 8. 9. 10 11 12 13 14 A chemist prepares a solution of potassium iodide (KI) by measuring out 3.0 × 10“ umol of potassium iodide into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in umol/L of the chemist's potassium iodide solution. Round your answer to 2 significant digits. alo Ar μ mol x10 Submit Assignment Continue 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility Show All IMG-4472.jpg IMG-4471.jpg IMG-4467.jpg IMG-4464.jpg IMG-4474.jpg MacBook Air DII F12 80 F9 F10 F11 F7 F8 F5 F6 F3 F4 F2 %23 $ 4 5 6 7 8 9 3 P 4.arrow_forwardIf an automobile travels 225 mi with a gas mileage of 20.5mi / gal,how many kilograms of CO{2} are produced? As- sume that the gasoline is composed of octane, C8H18(l) whose density is 0.69g / mL (b) Repeat the calculation for a truck that has a gas mileage of 5mi /galarrow_forward49.62 kcal into joulesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY