
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:Bin
e.net
Learning: Molecular Geometry and Bonding
land: Theories
na's
Dead
mpti...
A
-pex
gends
rome
EPIC
GAMES
Dynamic Study Modules - Learning: Molecular Geometry and Bonding Theories - Google Chrome
kf1.amplifire.com/amp/#s/learn-app/hf/assignment/BNWLMTL97
ic Games
auncher
©@
GeForce
experience
iCUE
Pearson
Terraria
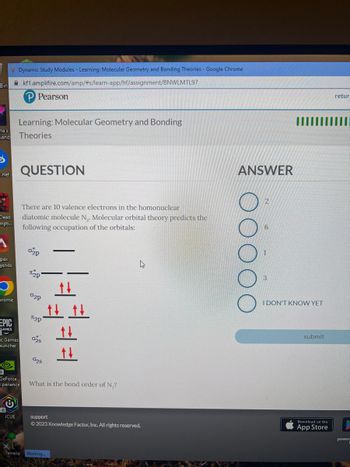
QUESTION
There are 10 valence electrons in the homonuclear
diatomic molecule N₁. Molecular orbital theory predicts the
following occupation of the orbitals:
02p
2p
02p
2p
025
028
tt
tt t
==
What is the bond order of N₂?
Waiting...
4
support
Ⓒ2023 Knowledge Factor, Inc. All rights reserved.
ANSWER
00
I DON'T KNOW YET
submit
Download on the
retur
power
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardUse the following Molecular Orbital Diagrams to answer questions 4 and 5. 4. Which of the following is paramagnetic? A. 02 2- B. Ne2 2+ C. 02 2+ D. F2 2+ E. None of the above is paramagnetic. 5. Which of the following is the MOST stable? A. C2 2+ B. N2 2+ C. B2 D. C2 2- E. B2 2+arrow_forwardPick the pair of possible bond angles in the Lewis structure below. a-90 or 180 b-109 or 120 c-109 or 120 d-120 or 180 e-90 or 120 f-109 or 180arrow_forward
- Macmillan Learning A 3D representation of a cyclohexane (C6H₁₂) molecule, a cyclic compound used in the manufacture of nylon and found in the distillation of petroleum, is shown. Name the geometry around each carbon atom. geometry: What is the hybridization of each carbon atom? O sp O sp³d² 200 sp² sn3³d ✓ O C OH Q Q ST Aarrow_forwardThis is not a graded question! Please help.arrow_forward6cdarrow_forward
- what bond angle is predicted for a liners molecule? which molecules were linear, did the molecular mechanics calculation product the correct bond angle list includes, CH4, CO2, H2CO, HCN, NH3, H2O, C2H4arrow_forwardPlease can you check my work thank youarrow_forwardIdentify the hybridization state of each of the indicated atoms CH, 1 3. 1 = sp2 2 = sp3 3 = sp3 O1= sp² %3D 2 sparrow_forward
- Please answer and be a hundred percent confident in your answerarrow_forwardDescribe the atomic hybrid orbitals that make up the C-N1 o bond in methyl azide, shown below. What about the N2-N3 T bond? (any nonbonding pair of electrons have been omitted) N, H3C° 2 N. 3arrow_forwardWhat is the most accurate bond angle approximation in a trigonal pyramidal molecule such as ammonia? Question 13 options: 107.3° 104.5° 120° 112.5° 90°arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY