
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
need help to figure out chart :(
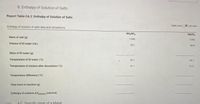
Transcribed Image Text:B. Enthalpy of Solution of Salts
Report Table CA.2: Enthalpy of Solution of Salts
Enthalpy of solution of salts data and calculations
Table view
List view
NH,NO,
Mgso,
Mass of salt (g)
1.990
1.992
Volume of DI water (mL)
50.1
500
Mass of DI water (g)
Temperature of DI water ("C)
24.1
24.1
Temperature of mixture after dissolution (C)
21.
31.0
Temperature difference ("C)
Total mass in reaction (g)
Enthalpy of solution AHolution (cal/mol)
G. Specific Heat of a Metal
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- if an acidic functional group is added with a basic functional group, the resulting structure will always be a neutrally-charged molecule provide logical reasoning that this is truearrow_forwardGive detailed Solution with explanation needed ...don't give Handwritten answerarrow_forwardTab Window Help Learning Xx AT HOMEWORK Module 9 X M hydrochloric acid Submit Answer MindTap Cengage Learning mentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786068&snapshotld-33225... References Use the References to access important values if needed for this question. : Concentration Acid/Base: This is group attempt 1 of 10 tv An aqueous solution of hydrochloric acid is standardized by titration with a 0.188 M solution of barium hydroxide. X If 23.6 mL of base are required to neutralize 16.7 mL of the acid, what is the molarity of the hydrochloric acid solution? G What volume (in mL) of a 0.125 ☆ Autosaved at 10:58 PM Q A ·arrow_forward
- Answer the following questions based on nitrous acid. a) Name the hybridization of the circled N. b)Write the O–N–O bond angle. c) Name the atomic (or hybrid atomic) orbitals used to form the N=O sigma and pi bonds. d) Name the atomic (or hybrid atomic) orbitals used to form the O–H sigma bond. Answer the following questions based on formic acid. a) Name the hybridization of the circled O b) Write the O–C–O bond angle. c) Name the atomic (or hybrid atomic) orbitals used to form the C=O sigma and pi bonds. d) Name the atomic (or hybrid atomic) orbitals used to form the C–H bond sigma bond.arrow_forwardDetermining the Acid, Base, Conjugate Acid, and Conjugate Base in a Reaction Label the acid and base, and the conjugate acid and base, in the following reaction. Use curved arrow notation to show the movement of electron pairs.arrow_forwardcan u lable the conugated base ad conugated acid pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY