
For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms.
You may assume all
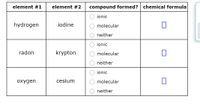
Given : Different elements.
To find : type of compound formed and chemical formula of compounds
Solution : As we know that, generally ionic compound are formed by the one metal and an non-metal that mean when a metal combine with non-metal then ionic compound formed.
While when a non-metal combine with another non metal then a molecular compound formed. But as we know, noble gases are inert gas they did not react so, noble gases (except Xenon) doesn't form neither ionic compound nor molecular compound.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

- www TT In the first column type the number of atoms in the covalent compound. In the second column, type the total number of VALENCE electrons in the compound (all electrons: shared and unshared electrons). In the third column, determine the total number of possible chemical bonds in the compound. Compound H₂ Cl₂ 0₂ N₂ H₂O SF2 CH4 NH3 PF3 CC14 C₂H6 CHEMISTRY Covalent Compounds and Electrons Number of Atoms I Total Valence Electrons Total possible of chemical bondsarrow_forwardWhat are covalent bonds? choose from the choices below: Bonds between atoms consisting of a shared electron pair. Bonds between a cation and an anion. Bonds between atoms of the same electronegativity. Bonds between identical atoms.arrow_forwardWhich of the following statements is true for an ionic bond? in the picture.arrow_forward
- For each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 barium sulfur hydrogen carbon bromine chlorine element pair will form a molecular compound C 0 molecular compound chemical formula 7 name 0 0 X Śarrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element pair molecular compound will form a element #1 element #2 molecular chemical name compound formula hydrogen fluorine sodium carbon carbon brominearrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element pair will form a alo molecular compound element #1 element #2 molecular chemical name compound formula Ar hydrogen fluorine sodium fluorine nitrogen fluorinearrow_forward
- Group 1 elements have an average electronegativity of 0.84 (not including hydrogen). Group 17 elements have an average electronegativity of 2.99. These two groups often form bonds. Given this information, which kind of bond will they form? * Periodic Table of the Elements He Li Be C Ne Na Mg Al SI CI Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd in Sn Sb Xe Hf Ta Re Os Ir Pt Au Hg TL Pb Po At Rn Cs Ba Fr Ra Db Sg Bh Hs Mt Ds Rg Cn Nh FL Mc Lv Ts Og Rf Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Cf Es Fm Md No Lr Ac Th Pa Np Pu Am Cm Bkarrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element pair alo molecular compound will form a element #1 element #2 molecular chemical name compound formula Ar carbon iodine hydrogen bromine lithium охудеnarrow_forwardUse the References to access important values if needed for this question. Complete the following structural formula for a neutral molecule by adding H atoms to complete the valence of each atom. Do not introduce any double or triple bonds. C-C-o Then write the molecular formula in the order CHO.arrow_forward
- For each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 sodium carbon hydrogen fluorine bromine bromine element pair will form a molecular compound molecular compound chemical formula 0 0 name 0 П 0 Śarrow_forwardWhich of the following statements about the ionic bond in ammonium chloride is correct? It is formed by the transfer of electrons from metal atoms to non-metal atoms. It is the electrostatic attraction between ammonium ions and chloride ions. It is formed by the sharing of electrons between the nitrogen atom and chlorine atom. There is actually no ionic bond in ammonium chloride.arrow_forwardFor the following ionic compounds, draw the Lewis symbols for the elements and, using arrows, show how electrons are transferred to make the ionic compounds. Also show the final ions formed and the formula of the ionic compound. a) aluminum chloride b) barium sulfidearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





