
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please,don't provied handwriting solution.

Transcribed Image Text:Propose a synthetic route with as few steps as possible to carry out the following
transformations.
Mention the reactions used.
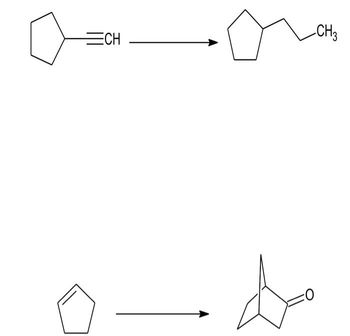
Transcribed Image Text:ECH
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If 100 mL of a 1:1500 methylbenzethonium chloride lotion is diluted with an equal volume of water, what will be the ratio strength of this dilution? a 1:3000 b 1:15000 c 1:100 d 1:67 e 1:6000arrow_forwardThe slope for the line is 71.22. Use the Beer's Law plot provided to determine the concentration for a solution with absorbance = 0.8829.arrow_forwardThe spectroscopic data in the table is generated with five solutions of known concentration. Concentration (M) 0.0179 0.0358 0.0716 0.143 0.286 Absorbance 0.1425 0.1896 0.5722 1.126 2.110arrow_forward
- Sodium + Silver chloride = ANSWER IN WORDS ONLY!!!arrow_forwardHello, please answer the following attached Chemistry question correctly and fully based upon the attached table. Please "Explain your answer" as stated. Thank you. Question: "Based on the class average from Table 3-1, what is the optimal pH for beets? Explain your answer.".arrow_forwardKOH, CH3CH₂OH reflux Atoms, and F Draw or taarrow_forward
- Hello I need help on these two quick questions. Thank you.arrow_forwardHelp me solve this, please? With the following data, and given the absorbance of the unknown solution is .502, calculate the concentration (M) of the unknown solution. There is data attached.arrow_forwardem1.png chem3.png Part 3: Q9 Average absorbance Flask 8 Flask 9 Flask 10 Flask 11 Part 3: Q9 Flask 8 Flask 9 Flask 10 Flask 11 Part 3: Q10 Flask 8 Flask 9 Flask 10 Flask 11 Q Part 3 0.146 [Fe] -0.002mol/L 0.166 volume 5.00mL 0.215 0.294 [FESCN mol/L) ✪ 4.59* 10 5.18*10* 6.64 x 10 9.08*10* [Fe(mol/L) 5.00 x 10 5.00*10* Y=3362x -0.0083 R¹ 0.9993 5.00 x 10 5.00*10* (Amounts of flask 8 (9 - 11's values are in the spreadsheet) 1:1 Ratio so, Molarity of Fe(NO3))3 = 0.2M Volume of Fe(NO3))3 = 5.00mL 71% Moles of Fe³= Molarity x volume(L) Moles of Fe = 0.2M × 0.005L = 1× 10-³mole 68% [SCN ](mol/ 4 x 10-4 5 x 10-4 7*10* 1*10* الخا chem2.png Concentration of Fe³ 9, X = mole of Fe³1x10³ mole Volume 0.0054 Ⓒ 8, Average absorbance for solution 8 (same for 9 - 11) Average absorbance for flask 8 = sum of all absorbances / number of readings (0.145 +0.147 +0.148) 3 Using equation of calibration curve's line(slope) of best fit, the average absorbances of the unknown concentrations to determine…arrow_forward
- Wrong solution aboarrow_forwardplease write clearlyarrow_forwardThe spectroscopic data in the table is generated with five solutions of known concentration. Concentration (M) 0.0133 m= 0.0266 0.0532 0.106 0.213 Absorbance 0.1271 What is the intercept of the linear regression line? 0.08531 0.5388 1.069 Use a spreadsheet program, such as Microsoft Excel, to graph the data points and determine the equation of the best-fit line. 1.954 What is the slope of the linear regression line formed by these points? M-1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY