
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
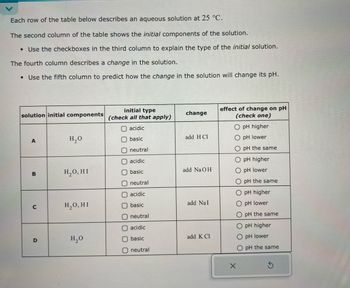
Transcribed Image Text:Each row of the table below describes an aqueous solution at 25 °C.
The second column of the table shows the initial components of the solution.
• Use the checkboxes in the third column to explain the type of the initial solution.
The fourth column describes a change in the solution.
• Use the fifth column to predict how the change in the solution will change its pH.
solution initial components
initial type
(check all that apply)
change
O acidic
A
H₂O
basic
add H Cl
O neutral
acidic
B
H₂O, HI
basic
add NaOH
O neutral
acidic
C
H₂O, HI
basic
add NaI
neutral
acidic
effect of change on pH
(check one)
O pH higher
O pH lower
O pH the same
O pH higher
O pH lower
O pH the same
O pH higher
O pH lower
O pH the same
O pH higher
D
H₂O
basic
add K Cl
O pH lower
O neutral
O pH the same
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Each row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type (check all that apply) effect of change on pH (check one) solution initial components change acidic pH higher H,0, H C104 add K C104 A basic pH lower neutral pH the same acidic pH higher В H,0 basic add Na Br pH lower neutral pH the same acidic pH higher H,0, H CIO, basic add KOH pH lower neutral pH the same acidic pH higher H,0 basic add Na OH pH lower neutral pH the same O O OO O O|O O O O O 0 0 0arrow_forwardConsider the following data on some weak acids and weak bases: name acid hydrocyanic acid nitrous acid solution 0.1 M KCN 0.1 M HONH3Br 0.1 M NaNO2 0.1 M KBr formula HCN HNO2 Ka 4.9 × 10 4.5 x 10 X - 10 PH choose one ✓ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. choose one ✓ choose one choose one name S base formula hydroxylamine HONH₂ ethylamine C₂H5NH₂ | K₂ 1.1 × 10 6.4 × 10 8 4arrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type solution initial components (check all that apply) O acidic A B с D H₂O H₂O, KOH H₂O H₂O, KOH basic neutral acidic basic neutral acidic basic neutral acidic basic ☐ neutral 00 change add NaC104 add KC1 add NaOH add HCl effect of change on pH (check one) O pH higher O pH lower O pH the same O pH higher O pH lower O pH the same O pH higher O pH lower O pH the same O pH higher O pH lower O pH the same ? olo Ararrow_forward
- Calculate the pH of the resulting solution if 34.0 mL of 0.340 M HCl(aq) is added to 44.0 mL of 0.340 M NaOH(aq). pH = Calculate the pH of the resulting solution if 34.0 mL of 0.340 M HCl(aq) is added to 24.0 mL of 0.440 M NaOH(aq). pH = OCT MacBook Pro & Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemistry | Publisher: University Scienarrow_forwardWrite the proton condition and acid/base mass balance equation for each of the following systems.arrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type (check all that apply) effect of change on pH (check one) solution initial components change acidic pH higher Н,О, КОН add HNO3 рH lower A basic neutral pH the same acidic pH higher Н, О, КОН add KNO3 рH lower В basic neutral pH the same acidic pH higher H,0 basic add HCl pH lower neutral pH the same acidic pH higher D H,O basic add NaCl рH lower neutral pH the same O OlO Oarrow_forward
- Each row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. solution initial components initial type (check all that apply) change effect of change on pH (check one) acidic A H₂O basic add NaNO3 neutral acidic O pH higher pH lower pH the same O pH higher B H₂O, HCI basic add K Cl O pH lower neutral acidic C H₂O, HCI basic add KOH neutral acidic D H₂O basic add HNO3 Oneutral O pH the same O pH higher O pH lower O pH the same O pH higher O pH lower O pH the same 5arrow_forwardThe pH readings for wines vary from 2.1 to 3.1. Find the corresponding range of hydrogen ion concentrations. 7.94 × 10−4 ≤ [H+] ≤ 7.94 × 10−3 3.1 × 10−4 ≤ [H+] ≤ 2.1 × 10−3 7.94 × 10−11 ≤ [H+] ≤ 7.94 × 10−10 3.1 × 10−11 ≤ [H+] ≤ 2.1 × 10−10 None of thesearrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type solution initial components (check all that apply) acidic basic neutral acidic basic neutral acidic basic neutral acidic basic neutral A B 09 C D H₂O H₂O H₂O, HCIO H₂O, HCIO4 0000000 change add HI add KI add NaClO add NaOH effect of change on pH (check one) O pH higher O pH lower OpH the same O pH higher O pH lower OpH the same O pH higher O pH lower O pH the same O pH higher O pH lower O pH the samearrow_forward
- Characterize the chemical shown below in terms of polarity and volatility. Assume a pH of 7. The structure drawn may NOT represent its ionization state at this pH. OH The chemical is nonpolar and volatile The chemical is nonpolar and not volatile O The chemical is polar and volatile The chemical is polar and not volatile The chemical is a cation and not volatile The chemical is an anion and not volatilearrow_forward5arrow_forward1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY