
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
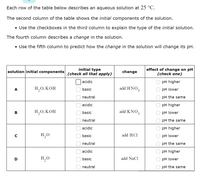
Transcribed Image Text:Each row of the table below describes an aqueous solution at 25 °C.
The second column of the table shows the initial components of the solution.
• Use the checkboxes in the third column to explain the type of the initial solution.
The fourth column describes a change in the solution.
• Use the fifth column to predict how the change in the solution will change its pH.
initial type
(check all that apply)
effect of change on pH
(check one)
solution initial components
change
acidic
pH higher
Н,О, КОН
add HNO3
рH lower
A
basic
neutral
pH the same
acidic
pH higher
Н, О, КОН
add KNO3
рH lower
В
basic
neutral
pH the same
acidic
pH higher
H,0
basic
add HCl
pH lower
neutral
pH the same
acidic
pH higher
D
H,O
basic
add NaCl
рH lower
neutral
pH the same
O OlO O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Each row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. alo initial type solution initial components (check all that apply) effect of change on pH change (check one) O acidic pH higher H,0 basic add H C10, O pH lower A O neutral O pH the same acidic O pH higher H,0 add Na ClO, B O basic O pH lower O neutral pH the same O acidic pH higher Н, 0, КОН V basic add H Br O pH lower O neutral O pH the same O acidic O pH higher D Н,о, КОН O basic add K Br O pH lower O neutral pH the same Explanation Check © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardEach row or the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. dlo initial type (check all that apply) effect of change on pH (check one) solution initial components change O acidic рH higher H,0, HI add Nal A basic pH lower neutral pH the same acidic pH higher B H,0 basic add K Br pH lower neutral pH the same acidic рH higher H,0 basic add KOH pH lower neutral pH the same acidic рH higher H,0, HI D basic add Na OH pH lower neutral pH the same O OO O OO O O O Oarrow_forwardwhat liquid does an antacid dissolve fater hot water, room temp water or room temp vinegar?arrow_forward
- Please help with the followingarrow_forwardWhich solution will contain the higher concentration of iodide ions? 0.25 mol/L calcium iodide or 0.45 mol/L potassium iodide Question 8 options: potassium iodide calcium iodide They contain the same concentration of iodide ions. Neither solution will contain iodide ions. This must be determined experimentally.arrow_forwardWhat is the pOH for a solution at 25 °C that has a H3O+ concentration of 6.57 ×10-6 M? A 34.1 % (NH4 )2SO4 (molar mass = 132.1 g mol−1) has a density of 1.15 g mL−1. What is the molarity of this solution? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 120. and -123 and 123. and 12.3) Determine the boiling point of a solution that contains 78.5 g of compound W (molar mass = 132.5 g mol–1) dissolved in 1088.6 g benzene (C6H6; molar mass = 84.156 g mol–1; Kb = 2.53 °C m–1; boiling point of pure benzene = 80.1 °C). (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 120. and -123 and 123. and 12.3) NEED HELP ASAP NO WORK NEEDEDarrow_forward
- What are the ingredients in carbonated soda responsible for the color change in litmus paper?arrow_forwardthe percentage of KHP in an unknown sample was determined. Which of the following is NOT true. Aqueous NaOH should be standardized so concentration is accurately known. The moles of KHP can be determined at the endpoint, which is close to the equivalence point. Percentage of KHP, is equal to the mass of KHP found, divided by the mass of the unknown, multiplied by 100.arrow_forwardWhich of the following would increase how quickly a solid will dissolve into solution? Increasing the temperature Increasing the particle size Decreasing the temperature Decreasing the pressurearrow_forward
- Recognizing common acids and bases Some soluble compounds are listed in the table below. Complete the table by filling in the name or chemical formula of each compound, whichever is missing. (If there is more than one way to name the compound, choose the name used when the compound is dissolved in water.) Also classify the compound using the checkboxes. compound NaI 0 12 sulfuric acid HCH₂ CO₂ ammonia name type of compound (check all that apply) strong weak strong weak acid acid base base ionic molecular O O O O O O × 3/5 00 Sarrow_forwardOver the course of a reaction, to reach equilibriuma) The molarities of the reactants and products will decreaseb) The molarities of the reactants will increase and the molarities of the products will decreasec) The molarities of the reactants and products will increased) The molarities of the reactants will decrease and the molarities of the products will increase.arrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type solution initial components (check all that apply) change effect of change on pH (check one) acidic pH higher H₂O A basic add KNO3 pH lower neutral pH the same acidic pH higher B H₂O, NaOH basic add HClO4 pH lower neutral pH the same acidic pH higher C H₂O basic add KOH pH lower neutral pH the same acidic pH higher H₂O, NaOH basic add NaC104 pH lower neutral pH the samearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY