
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
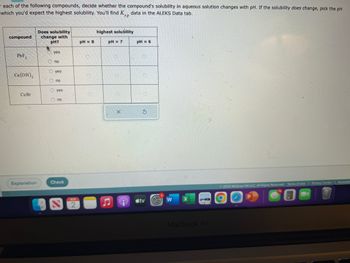
Transcribed Image Text:each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH
which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab.
sp
compound
PbF₂
Ca(OH)₂
Cu Br
Explanation
Does solubility
change with
pH?
. yes
Ono
yes
no
O yes
O no
Check
AUG
2
pH = 8
highest solubility
pH = 7
X
e
pH = 6
5
tv
W
X
MacBook Air
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessib
70
P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. sp highest solubility Does solubility change with compound pH? PH = 6 pH = 7 pH = 8 yes BaCO3 no yes Ar Cal2 no yes Cu Br no X Ś ?arrow_forwardA solution contains 1.18x10-2 M potassium chromate and 6.21x10-3 M sodium bromide. Solid silver acetate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of silver ion when this precipitation first begins? [Ag+] = Marrow_forwardIf 200 mL of 1.0 x 10^-8 M AgNO_3 is mixed with 200 mL of 1.0 x 10^-8 M Nal, what will occur? For Agl, Ksp = 8.5 x 10-17, Sodium iodide will precipitate. Sodium nitrate will precipitate. No precipitate will form. Silver iodide will precipitate Silver nitrate will precipitate.arrow_forward
- Calculate the solubility at 25 °C of Ni(OH)2 in pure water and in a 0.0170M NaOH solution. You'll find K sp Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0170 M NaOH solution: 0- 00 x10 X ☐☐ 5 010 data in the ALEKS Data tab.arrow_forwardPlease don't provide handwritten solution ....arrow_forwardFor each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. sp Does solubility change with pH? highest solubility compound pH = 5 pH = 4 pH = 3 Ca, (PO.), yes O no O yes AgCN O no yes PbCl2 no Explanation Check Accessibili O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy 17 MacBook Air F10 F9 F7 F8 o oo oarrow_forward
- Barium hydroxide, Ba(OH)2, dissolves in water to the extent of 18.5 g per liter. What is the value of Ksp for Ba(OH)2 ? Ba(OH)2(s) ⇒ Ba²+ (aq) + 2 OH¯(aq) K sp =arrow_forwardFor each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K,, data in the ALEKS Data tab. compound AgCl Ca, (PO₂)₂ Ba(OH)₂ Does solubility change with pH? yes Ono Oyes O no Oyes O no pH = 5 O highest solubility pH = 6 O O X pH = 8 O ?arrow_forwardtab esc For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. sp compound 1 CaBr, CaCO3 Ca (OH)₂ Q Does solubility change with pH? 2 O yes O no O yes no -0. F2 yes W no # 3 pH = 7 80 F3 E highest solubility pH = 8 $ 4 X 000 F4 R pH = 9 % 5 S F5 T MacBook Air < F6 Y & 7 AA F7 U 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility * 00 8 DII F8 9 Save For Later F9 Submit Assignment F10 P F11 + 11 { [ 000 18 Ar F12 ?arrow_forward
- For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. sp Does solubility change with pH? highest solubility compound pH = 5 pH = 6 pH = 8 O yes Ba(OH), O no do O yes CuBr O no O yes AgCN O no Explanation Check Rights Reserved Terms of Use Privacy Accessibility O 10:00 O oo o0arrow_forwardA solution contains 0.021 M Cl- and 0.017 M I. A solution containing copper (I) ions is added to selectively precipitate one of the ions. At what concentration of copper (I) ion will a precipitate begin to form? What is the identity of the precipitate? Ksp(CuCl) = 1.0 × 10-6, Ksp(Cul) = 5.1 × 10-12arrow_forwardFor each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. sp compound Cu Br Zn S Cal₂ Does solubility change with pH? ο οίο ο yes no yes no yes no pH = 4 highest solubility pH = 5 X pH = 6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY