
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
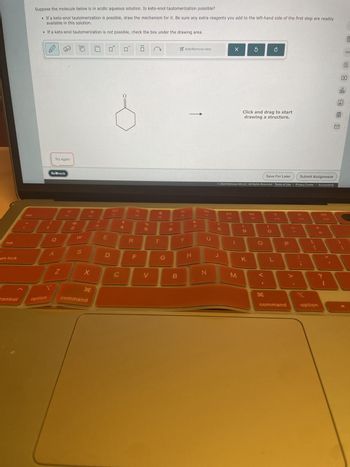
Transcribed Image Text:È
Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible?
• If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand side of the first step are readily
available in this solution.
• If a keto-enol tautomerization is not possible, check the box under the drawing area.
tab
GSC
ps lock
Try again
Retheck
+
A
02
Z
43
80
Add/Remove step
54
LU
W
E
S
F4
R
%
205
F5
T
<6
D
F
G
T
H
command
Control
option
ㅇ
FG
Y
87
&
8
00-
olo
Ar
Click and drag to start
drawing a structure.
Save For Later
Submit Assignment
© 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
4
F7
F10
FB
H
כ
Ո
00
8
の
J
K
L
N
C
V
B
M
H
ין
P
F11
command
F12
+ //
option
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Complete the reaction below. 1) What are the names of the starting compound and product? 2) Draw out a COMPLETE mechanism and be sure to use arrows and appropriate charges. Но на илон ля Sulfuric Acid, ???arrow_forwardplease answer part c and d!arrow_forwardWhat are the reagent to get the product. Please include mechanismarrow_forward
- 3. Provide the mechanism for the following reversible nucleophilic addition. Show all curved arrows and intermediates. cat. H+ H₂O HO OH H H 1) protonate to activate 2) nucl adds 3) deprotonate what staysarrow_forwardConsider the SN2 reaction of butyl bromide with OH- ion. CH3CH2CH2CH2B + OH- → CH3CH2CH2CH2OH + Br Assuming no other changes, what effect on the rate would result from simultaneously doubling the concentrations of both butyl bromide and the OH- ion? Draw the curved arrow mechanism for the SN1 reaction of methanol with tert-butyl iodide. Next, draw an Energy vs. Reaction Coordinate diagram for this reaction, labeling all transition states as "TS" and intermediates as appropriate for those drawn in the mechanism above, showing the relative energies on the diagram.arrow_forwardDraw a step-wise mechanism for the following substitution reaction. Be sure to add lone pairs and charges where relevant.arrow_forward
- Select one deactivator & meta director to be on a benzene ring to start. You will then add a bromine to this group showing the COMPLETE mechanism, including all resonance structures. Put a box around the stabilizing resonance structure and explain why it is stabilizingarrow_forward[Review Topics] [References] Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. H3C CH3 H2O NaOH • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Nat, I', in your answer. • In cases where there is more than one answer, just draw one. C opy aste C. Previous Next Email Instructor Save and Ex Cengage Learning | Cengage Technical Support 5:48 PI 82°F 3/28/20arrow_forwardPlease help answer the attached question...thank you!arrow_forward
- Select one deactivator & ortho para director to be on a benzene ring to start. You will then add a bromine to this group showing the COMPLETE mechanism, including all resonance structures. Put a box around the stabilizing resonance structure and explain why it is stabilizing.arrow_forwardplease help me with all parts of this question, it is a single question with multiple subpartsarrow_forwardGive detailed Solution with explanation needed..give correct answer if you not then don't give answer..I will give you upvote..draw out all of the substitution and elimination reactionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning