Question
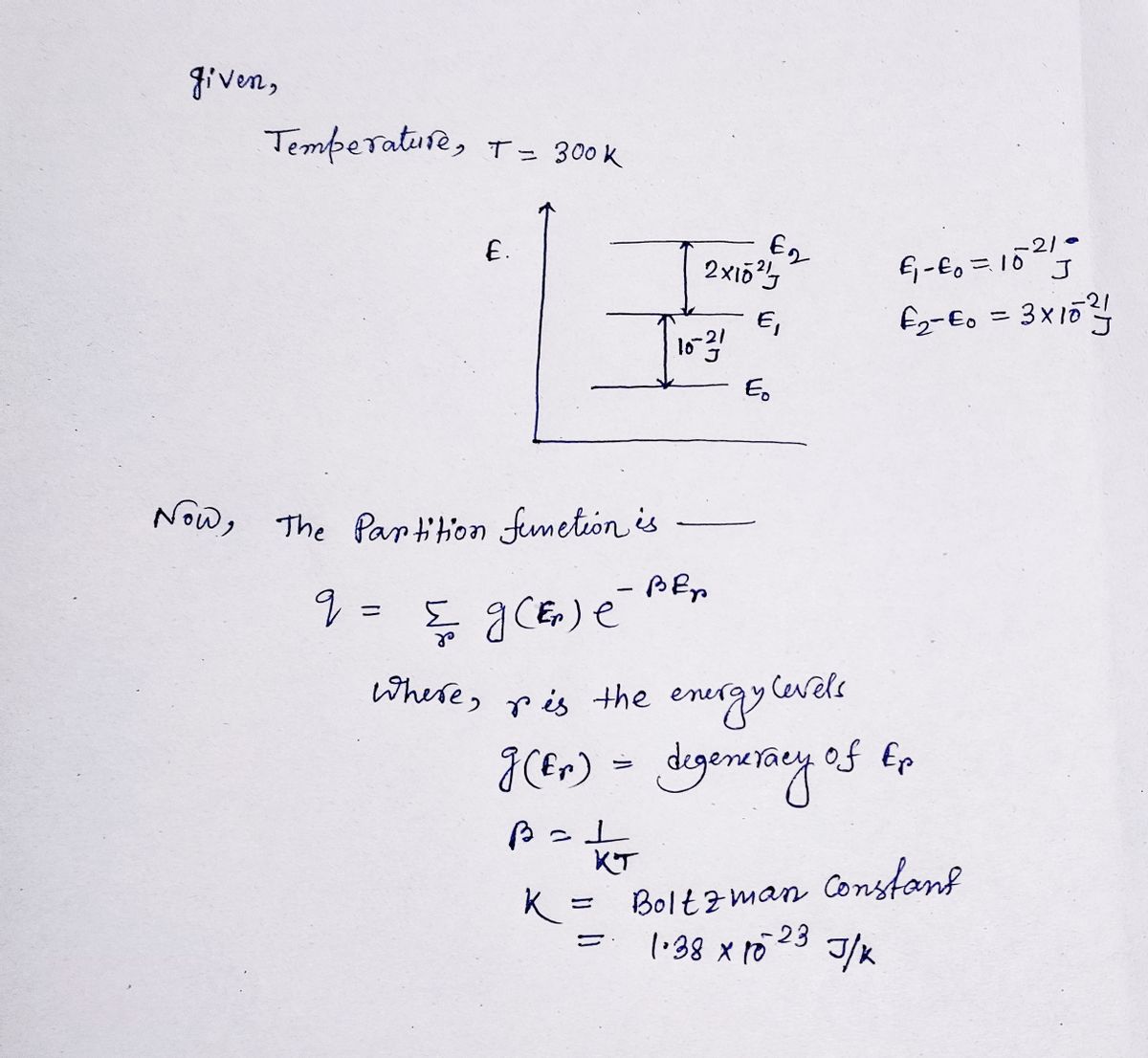
Calculate the partition function for the following system at 300 K.
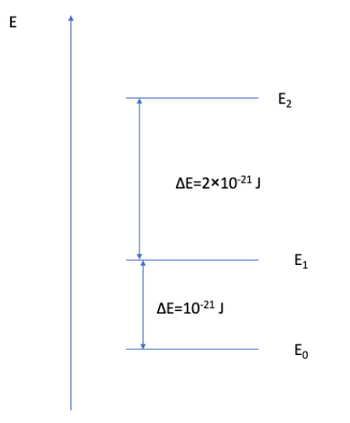
Transcribed Image Text:E
△E=2×10-21 J
DE=10-21 J
E2
E1
Eo
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Similar questions
- Derive the heat capacity Cv of a system whose canonical partition function is Z = AVNT³N where A is a temperature-independent constant.arrow_forwardLearning Goal: To derive the expression for the work done by an expanding gas, W pAV, and to understand how it follows from the expression WFAz for mechanical work In thermodynamics, positive work is defined to be the work done by a system on the exterior world. In classical mechanics, the converse is true: One always considers the work done on a system by the outside world to be positive. For example, suppose you push a large block with a certain force of Figure 101 Gas Part C What is AV, the increase in volume of the gas? Express the increase in volume in terms of Ar and other given quantities. >View Available Hint(s) - ΑΣΦ AV - PAAx Submit Previous Answers Request Answer * Incorrect; Try Again Part D Complete previous part(s) Part E Complete previous part(s) Part F Complete previous part(s) F 02-23-2arrow_forwardFor a p, v, T system formed by a mole, it has been empirically obtained that the internal energy per unit mass can be written in the form: u = apv+b, where a, b are constants. Find the equation for the adiabat in this system.arrow_forward
arrow_back_ios
arrow_forward_ios