
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I attached the question and the answer
How does the negative sign appear in the first integral
And then what are they doing in the second part what’s env ?
Shouldn’t we just find the total change in entropy
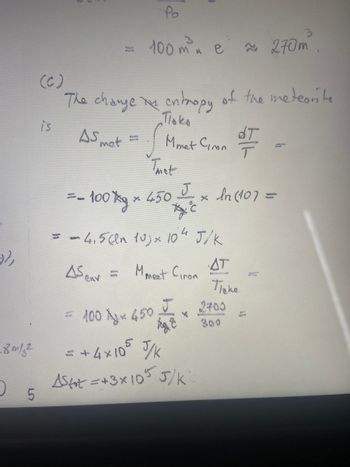
Transcribed Image Text:.8 m/s²
)
5
=
is
(C)
The change in entropy of the meteorite
Поко
Mmet Ciron
AS met
BON
Po
100 m²³ e 270m
= - 100 g *
Thet
J
Ac
= = 4,5 cm 10) × 104 J/K
As env=
M meet Giron
× 450
wome
× In (107 =
fog
= +4×105 J/K
Astot =+3x105 5/K
dT
T
AT
Паке
2400
= 100 × 450 × 2705
x
300
J

Transcribed Image Text:balloon
130
1.3
= balloon W/L
Po
3000/3x10²
be
100-e~ 270 m³
(c) A meteorite made of iron with mass Mmet = 100kg and initial temperature Tmet = 3000k, falls into a
large lake with temperature Thake = 300k. Estimate the total entropy change as the meteorite temperature
reaches the lake temperature (In (10)=2.3)
* The change of entropy of the meteorite
is AS met
Ticke
I
Tret
Mmet Ciron
drf
T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- How is the total work done by the reversible cycle related to the entropy change of the Universe.arrow_forwardExplain the Lee's disc method of measuring the thermal conductivity of a bad conductor with necessary sketches. Explain the following special cases of first law of thermodynamics with neat sketches. () Adiabatic processes (ii) Constant volume processesarrow_forwardPlease help mearrow_forward
- Write down the thermodynamic equations of state for the pressure and entropy which follow from the Helmholtz free energy F. Hence express the heat capacity at constant volume in terms of F. [You may use the definition: dF = −SdT – PdV.]arrow_forwardThe entropy S of a system of N spins, which may align either in the upward or in the downward direction, is given by S=-k,N|pln p+(1– p) In(1– p)] . Here k, is the Boltzmann constant. The probabinty of alignment in the upward direction is p. The value of p, at which the entropy is maximum, is (Give your answer upto one decimal place)arrow_forwardUsing molecular and system partition functions it is possible to derive the Sackur-Tetrode equation, which allows the computation of absolute entropy from molecular parameters: 2лmkвT S = {NkB + NkB In In [ (2TmkyT) 3/² V h² N In this equation, the quantity V/N is sometimes written as 1/p. = (a) Use this expression to compute the entropy due to translation for one mole of CO₂ gas at 400 K, for which mco2 = 44.01 Daltons, and its molar volume (pressure = 1 bar) is V 0.03326 m³. (Note: 1 Dalton = 1.66053 × 10-27 kg). Be careful with the computation- keep track of units and make sure that the argument of the LN function is unitless. Your answer should be between 100 and 200 J/K if you do everything correctly. (b) If the mass of neon were twice as large (88 instead of 44 Daltons), by how much would the extra mass affect its translational entropy? Report the ratio of the entropy with the heavier mass to that found in part (a).arrow_forward
- A plastic bag containing 0.2 kg of water at 20°C is dropped from a height of 0.5 m onto an insulating carpet. Assume that the bag does NOT break. What is the approximate probability that a similar bag sitting on a carpet will do the reverse; that is, spontaneously jump 0.5 m in the air? Express your answer in the form "Probability = 10-x," where x is a number you will calculate. (Hint: Note that ey = 10y÷ln(10).)arrow_forwardNumber 3 pleasearrow_forwardAnswer in 90 minutes please.arrow_forward
- For a dilute gas of N monatomic particles with mass m and total energy E, use the Sackur- Tetrode equation for the entropy S V = log + NkB to derive expressions for the pressure and internal energy in terms of the temperature T and volume V. [You may use that X₁ = 3πh² N/(mE).] tharrow_forwardPolymers, like rubber, are made of very long molecules, usually tangled up in a configuration that has lots of entropy. As a very crude model of a rubber band, consider a chain of N links, each of length L Imagine that each link has only two possible states, pointing either left or right. The total length L of the rubber band is the net displacement from the beginning of the first link to the end of the last link. Using the thermodynamic identity, you can now express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T , N, and l.arrow_forwardSuppose that 10 distinguishable particles are equipartitioned in a container that has 100 equal-sized compartments. What is the entropy on this system?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON