
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please provide detailed answers and proper arrows
thank you so much
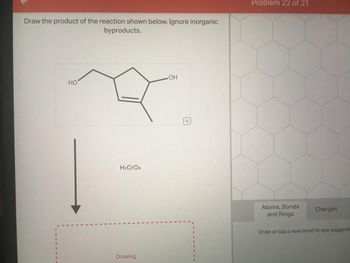
Transcribed Image Text:Draw the product of the reaction shown below. Ignore inorganic
byproducts.
HO
H₂CRO4
Drawing
.OH
Problem 22 of 21
Atoms, Bonds
and Rings
Charges
Draw or tap a new bond to see suggesti
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- AME M Inbox (1,600)-fantil@udeledu X Mail- Francesca A Tantillo-Out x Homepage - CHM150-251 Chen X + → Capp.101edu.co < A Z 89'1 Rain coming 2 S # 3 E D C Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. $ Which of the following best describes where fluorine can be found on the periodic table? 4 40 R % F 5 B) Noble gases A) Alkaline earth metals C) Lanthanides D) Actinides E) Halogens G O Question 19.b of 23 6 B Aktiv Chemistry OME V Y H * الات & 7 -0✓ INN N PrtScn * 8 J Home End o M ( O 9 K O ) 0 L Alt Pgup m P Pon 12 B ? □ ( 4D Update ***/***/// 512 PM D 7/6/2022 < X Submit Del Backspace Enter Shiftarrow_forwardPls answer this for me. Thank you so mucharrow_forward1.) CF3CO3H b.) d)arrow_forward
- CI OMe 1) NaNH2, NH3 (1) 2) H₂O*arrow_forwardC. Cr(C104)3 Show Hintarrow_forwardMyCSU - Columbus State Univer. x | № Inbox - bailes_amber@columbu: x D2L Homepage - Columbus State Un X - со A ALEKS-Amber Bail www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lJgXZp57itWHhRgilODc5MqvhZbKYx2-U-037007TYd Gmail YouTube Maps MyCSU - Columbus... Homepage - Georg... Microsoft Office Ho... B Lesson 2 Disc O CHEMICAL REACTIONS = Solving for a reactant using a chemical equation Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (1₂0). What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas? Be sure your answer has the correct number of significant digits. x10 g ANAKKALE X S ? Email 4 Jessy Vseforestainty a jedan den so $45******arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY