
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
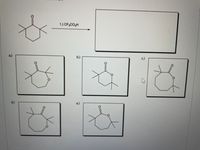
Transcribed Image Text:1.) CF3CO3H
b.)
d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. iv- v 2 last questionsarrow_forward2. C12H24(I) + O2(g)arrow_forwardComplete the reactions below with appropriate reaction conditions, make sure that your set of reaction conditions produce the indicated product as a major product. Please indicate clearly whether the reaction conditions occur in one or several distinct steps. Reaction Conditions Reactant Product a) Br b) OH c) HOarrow_forward
- C. CH3 CH,CH3 9. CH;CH, C-CH-CH-CH,CH,CH,CH,CH3 し。) CH,CH,CH3 1. b. CHCH3 CH3CH2 -CCHCH3 CH,CH3 Died in U.S.A. with U.S. and foreign parts Ohio 45429 patentsarrow_forward7) The most important reaction that allows our cars to do work is octane (CSH18) combustion 2 C8HI8 + 25 02 à 16 C02 + 18 H20 a) Theoretically speaking, if we fully combust 3.0 kg of octane, and keep the products at STR, what volume of gas do we expect to be produced? b) If the % yield for this combustion reaction is 90%, what is the actual volume? 8) Name two characteristics that describe real gases and not ideal gasses. 9) a) A climber takes a mole of ideal qgas to the top of Mount Everest, where the pressure is 304 torr and the temperature is subzero at -17° C. What is the volume of the gas? b) The gas taken to Mount Everest is oxugen gas. What is the root mean square velocity of gas molecules in that sample of gas? 10.) a) An 8.00L tank at 7.3° C is filled with 24 g of dinitrogen monoxide (N2O) and 8.2 g of sulfur hexafluoride gas (SF6). What is the mole fraction of sulfur hexafluoride? b) What is the ratio between the rates of effusion of sulfur hexafluoride and dinitrogen…arrow_forwardWhich molecule is a complete organic molecule (i.e. all atoms are shown correctly)? A H. c=C–C= C–H H C H. C=C-C-C-H | H. H. C H. C- D H. нн . C С —С—Н C=C–C- C-H H H. ОН Н А I-0-I エーO I-Ú-I I-U-I I-U-I エー○arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY