
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give typed full explanation
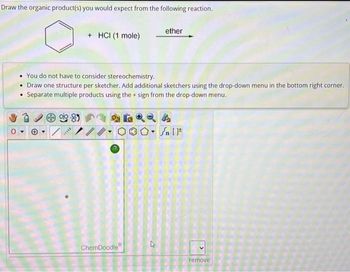
Transcribed Image Text:Draw the organic product(s) you would expect from the following reaction.
***
+ HCI (1 mole)
You do not have to consider stereochemistry.
Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
• Separate multiple products using the + sign from the drop-down menu.
8)
ChemDoodle
ether
4
Sn [F
remove
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please provide detailed solution and give explanation..arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardThis is not really a question but, I have a presentation to do on one of the following polymers. I was just wondering if you can pick the top 5 best out of them all. I will include the questions I will be researching, I just want an expert opinion on which polymer would be th best for these questions. :) These are the questions that I will be resaerching about: List its current uses and why we use it for those applications. What is it? What are its components (i.e., its monomers)? How is the polymer manufactured? What is the chemical reaction that occurs in its creation? What is the chemical reaction that occurs in its degradation? What are the environmental consequences of its production? What are the environmental consequences of its disposal? Propose a solution to the environmental consequences you listed above. For example, could something about the polymer be changed to alleviate the problems or could another, safer polymer be used in its place? And these are the polymers I…arrow_forward
- File Edit View History Bookmarks Profiles Tab Window Help 7 YouTube Maps = tab liquid x N. Ask Yox N. Ask Yo x A ALEKS X www-awu.aleks.com/alekscgi/x/1sl.exe/1o_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHintXeoleXL2H OSTATES OF MATTER Using heat of fusion or vaporization to find the heat needed to... esc Explanation 39 cto X 1 Q A 2 Gheat ox v Calculate the amount of heat needed to melt 88.1 g of solid methanol (CH3OH) and bring it to a temperature of -23.6 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. 0 Z Check W S 3 X g X E D $ 4 C S > R F % 5 I tv T V 6 G B Thank Y 7 H U G 111 ck X N The 8 © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 274@ A Aa O J Sketch X New 1 x + GCwaDdYUtqAJT1yasCs1fG_rUoyaRSVpsjfsOdH... ☆ 0 1 M 9 ( K O < 431% D ge Gwet dr x 0 L Wed 9:43 PM 0/5 P command Lara V ? 1 dlo Aarrow_forwardcollege.com/course.htm MS-¡PI... Give the correct IUPAC name for each of the following compounds. Submit Part C Request Answer CH₂ CH₂ CH₂ CH3 CH3CHCH₂CH CH3 CH₂CH3 Spell out the full name of the compound. Submit Request Answer P Pearson Review | Constants | Periodic Terms of Use | Privacy Policy | Permissions Contact Us Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use O 2 D 9:26 1 10/14/20arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY