
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
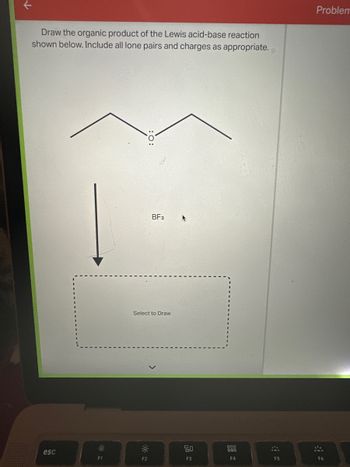
Transcribed Image Text:个
Draw the organic product of the Lewis acid-base reaction
shown below. Include all lone pairs and charges as appropriate.
esc
O
F1
BF3
Select to Draw
F2
90
F3
900
000
F4
F5
Problem
F6
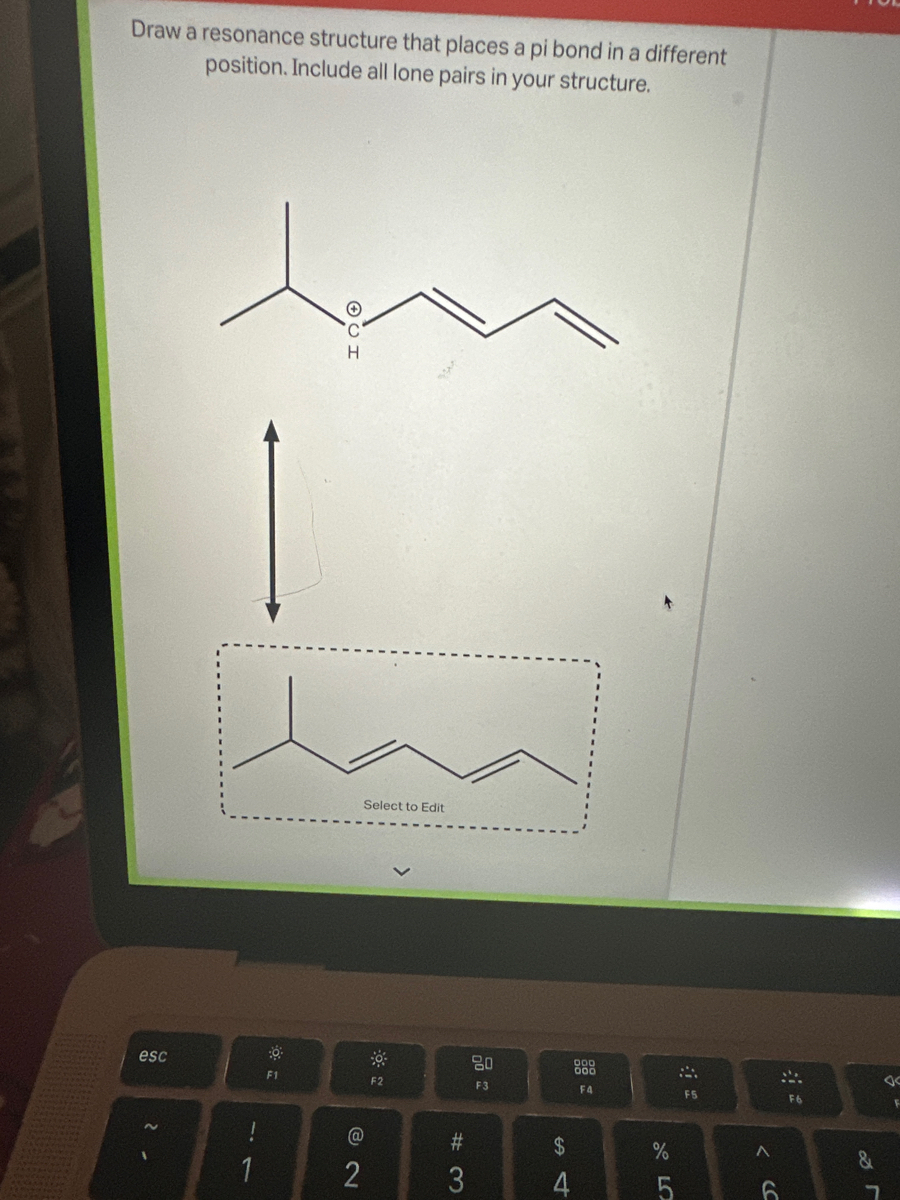
Transcribed Image Text:Draw a resonance structure that places a pi bond in a different
position. Include all lone pairs in your structure.
esc
2
!
F1
Select to Edit
@
2
F2
#3
3
20
F3
$
st
4
F4
%
LO
5
A
F5
A
6
F6
&
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please helparrow_forward7. Now circle the lewis acid and lewis base sites on the following compounds. Some compounds may have both or just one. Note you can use LA for lewis acid and LB for lewis base as labels. S-H H.arrow_forwardDraw the organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. HCI Drawing C:Oarrow_forward
- Draw the organic product of the Lewis acid-base reaction shown below. Include all lone pairs and charges as appropriate. :O: BF3 Select to Draw Please select a drawing or reagent from the question areaarrow_forwardDraw the major organic oduct of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. HO NaOH :0: -0:0 Qarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- 6. Rank the indicated a-H's from most to least acidic (most acidic= 1). Be sure to explain your rankings for each by drawing significant resonance structures for the conjugate base and commenting on any other factors that contribute to your rankings. H H H H. A B Cmpd Rank Resonance Structures Reasoning A Вarrow_forwardDraw the mechanism showing the Brønsted acid/base reaction between propanoic acid and methylamine. Label the acid, base, conjugate base, and conjugate acid. Show all curved arrows, lone pairs and nonzero formal charges. einedoelM HO, CH;NH, propanoic acid pKa = 4.9 methylamine Mechanism: Using the pKa information provided/pKa tables (table appended to the end of this document), determine whether the starting reagents (propanoic acid and methyl amine) or resulting products would be favored at equilibrium (no math needed!).arrow_forward#2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY