
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
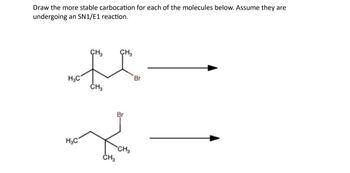
Transcribed Image Text:**SN1/E1 Carbocation Stability Analysis**
In this exercise, you are asked to draw the more stable carbocation for each of the molecules undergoing an SN1/E1 reaction. The stability of a carbocation is crucial in determining the rate of reaction in these mechanisms. Let's examine each molecule:
1. **Top Molecule:**
- **Structure:** A branched alkyl structure with a bromide leaving group attached to a secondary carbon.
- **Carbocation Formation:** The bromide ion leaves, resulting in a carbocation at the secondary carbon. The stability can be increased if the carbocation is adjacent to a tert-butyl group, allowing hyperconjugation and inductive effects to enhance stability.
2. **Bottom Molecule:**
- **Structure:** Another alkyl structure with a bromide leaving group, attached to a tertiary carbon this time.
- **Carbocation Formation:** The bromide ion leaves, forming a carbocation at the tertiary carbon, which is inherently more stable than secondary carbocations due to additional hyperconjugation and inductive effects from surrounding methyl groups.
**Explanation of Mechanisms:**
- **SN1 Reaction:** Involves two steps: formation of the carbocation and nucleophilic attack. The stability of the carbocation is a key factor in reaction rate.
- **E1 Reaction:** Also involves two steps: formation of the carbocation followed by elimination. The likelihood of elimination versus substitution often depends on the reaction conditions and the nature of the nucleophile/base.
These reactions emphasize the role of carbocation stability, which can be enhanced through molecular structure and neighboring electron-donating groups.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the skeletal structure(s) of the major product(s) of the reaction below. H Br₂ FeBrarrow_forwardList the following alkyl halides in order of decreasing reactivity toward SN1/E1 reactions (from 1: most reactive to 4: least reactive).arrow_forwardWrite an additional resonance contributing structure for each carbocation and state which of the two makes the greater contribution to the resonance hybrid.arrow_forward
- Draw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 H3PO4 HO, + H3C CH3 HO HO First stage in synthesis of the epoxy and polycarbonate ingredient bisphenol-A You do not have to consider stereochemistry. Include all valence lone pairs in your answer. In cases where there is more than one answer, just draw one.arrow_forwardComplete these SN2 reactions, showing the configuration of each product.arrow_forwardGive the product of the following reaction. NO₂ O O NO₂ NO₂ NO₂ Br Br Br Br₂ FeBr3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY