
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give detailed Solution with explanation needed ..don't give Handwritten answer
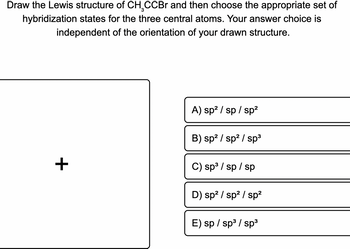
Transcribed Image Text:Draw the Lewis structure of CH₂CCBr and then choose the appropriate set of
hybridization states for the three central atoms. Your answer choice is
independent of the orientation of your drawn structure.
+
A) sp² /sp/sp²
B) sp² / sp² / sp³
C) sp³ / sp / sp
D) sp² / sp² / sp²
E) sp / sp³ / sp³
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation neededarrow_forwardWrite the ground-state electron configuration for each atomand ion pair-Zr, Zr2+, Co, Co2+, Tc, Tc3+, Os, Os4+?arrow_forwardBe sure to answer all parts.Using the periodic table only, arrange the members of each of the following sets in order of increasing bond strength:(a) Br―Br, Cl―Cl, I―I (b) S―H, S―Br, S―Clarrow_forward
- Aluminum chloride, AlCl₃, is used in a variety of organic reactions as a Lewis acid. Although it adopts a more complex geometry in the solid state, consider it as a single molecule (i.e., in the gas phase). Given the electron configuration of Al is [Ne]3s²3p¹, how many valence electrons does Al have?arrow_forwardHow can I use the Born Haber cycle to establish the lattice energy of CsCl (s)from the following data: ΔHf° [CsCl(s)] = -442.8 kJ/mol; enthalpy of sublimation of Cesium is 78.2 kJ/mol; enthalpy of dissociation of Cl2 (g) = 243 kJ/mol Cl2 ; IE1 for Cs(g) = 375.7 kJ/mol; electron affinity enthalpy-EA1 for Cl(g) = -349kJ/mol.arrow_forward2. For each pair, choose the compound with the smaller lattice energy, and explain your choice: (a) Cas or Bas (b) NaF or Mgoarrow_forward
- What is noble gas electron configuration of Zn2+ ?arrow_forwardConsider the following electron configuration:(σ3s)2(σ3s*)2(σ3p)2(π3p)4(π3p*)4Give four species that, in theory, would have this electron configuration.arrow_forwardUsing the concept of formal charge to prove your answer, indicate which of the following is the correct Lewis structure for CH4S? H. A) H S-C-H B) H C S-H H. STRUCTURE (A) STRUCTURE (B) CfC = CfC = %3D %3D Cf S = Cf S = %3D CORRECT STRUCTURE =arrow_forward
- 1- The following chemical reaction takes place in an aqueous solution: FeBr2 (aq) + 2KOH (aq) → Fe(OH)2 (s) + 2KBr (aq) Write the net ionic equation for this reaction. 2- Draw the Lewis structure for ethane (C2H6). Be certain you include any lone pairs. 3-Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HBr + Ca(OH)2 → 4-A chemistry student needs 25.0 g of methyl acetate for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the density of methyl acetate is 0.934 g *cm-3. Calculate the volume of methyl acetate the student should pour out. Be sure your answer has the correct number of significant digits. ____________mL 5- NASA communicates with the Space Shuttle and International Space Station using Ku-band microwave radio. Suppose NASA transmits a microwave signal to the Space Shuttle using radio…arrow_forwardUse the following data to estimate AH; for potassium bromide. K(s) + Br2 (9) - KBr(a) Lattice energy -671 kJ/mol Ionization energy for K 419 kJ/mol Electron affinity of Br -325 kJ/mol Bond energy of Br2 Enthalpy of sublimation for K 193 kJ/mol 90. kJ/mol AH = kJ/molarrow_forwardDraw the Lewis electron dot structures for these molecules, including resonance structures where appropriate:(a) CS32−(b) CS2(c) CS(d) predict the molecular shapes for CS32− and CS2 and explain how you arrived at your predictionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning