
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
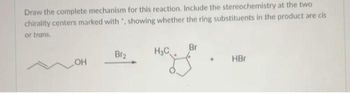
Transcribed Image Text:Draw the complete mechanism for this reaction. Include the stereochemistry at the two
chirality centers marked with ", showing whether the ring substituents in the product are cis
or trans.
OH
Br₂
H₂C
Br
HBr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The southern pine beetle utilizes a multi-component aggregation pheromone (one component shown below) to start mass colonization of healthy trees. The biosynthetic pathway involves the cyclization of this acetal from the straight chain structure. Draw the straight chain structure that could be used to form this acetal. Use wedges and dashes to correctly depict the stereochemistry. H3C- OH ✔arrow_forwardDraw the products of the following reactions, indicating both regiochemistry and stereochemistry when appropriate. Use wedge and hash bonds ONLY when needed to show reaction stereochemistry. In cases where there is more than one answer, just draw one.arrow_forwardTrans-1-bromo-2-methylcyclohexane will yield a non-Zaitsev elimination product (3-methylcyclohexene) upon reaction with KOH. Show this reaction by drawing the chair conformations of the reactant and product. Include the curved arrows and explain why the product is not a non-Zaitsev product.arrow_forward
- What is the major product of the following reaction? Chiral products are racemic. pressurearrow_forwardDraw the highest and lowest energy conformations. In cases where two or three conformations are degenrate, choose only one as your answer. Use Ch2Ch3, H and Ch3 for the structure.arrow_forwardRacemic compound with stereochemistry as shown Give the IUPAC name of the above structure in the box below:arrow_forward
- Please don't provide handwritten solution...arrow_forwardDraw the following molecule, don't forget stereochemistry : (E)-5-ethyl-5-methylhept-3-enearrow_forwardDraw all of the products, both constitutional isomers and stereoisomers, of the following reactions. a) 4,4-dibromo-1-ethylcyclopentene ---->Br2,H2O b) 3,3-dibromo-1-ethylcyclopentene ----> HBr c) 3,3-dibromo-1,2-dimethylcyclopentene ----> 1. BH3. 2. H2O2, NaOHarrow_forward
- Give the proper IUPAC name for the molecule shown as a Fischer projection. Make sure to include the correct (R) or (S) designation where appropriate. CH₂CH3 Br H CH₂CH₂CH3 The name of the molecule is: O (R)-4-bromohexane (S)-3-bromohexane (R)-3-bromohexane (S)-4-bromohexanearrow_forwardWhich of these common group names is ambiguous? You may neglect stereochemistry. O sec-hexyl O isopropyl O Neopentyl Q sec-butyl O more than one of these.arrow_forwardO || CH3-CO-C-CH3 tCH3CH2CH, NH CH 3 | CH3-C-CL + CH3-N-CH3 Complete the following reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY